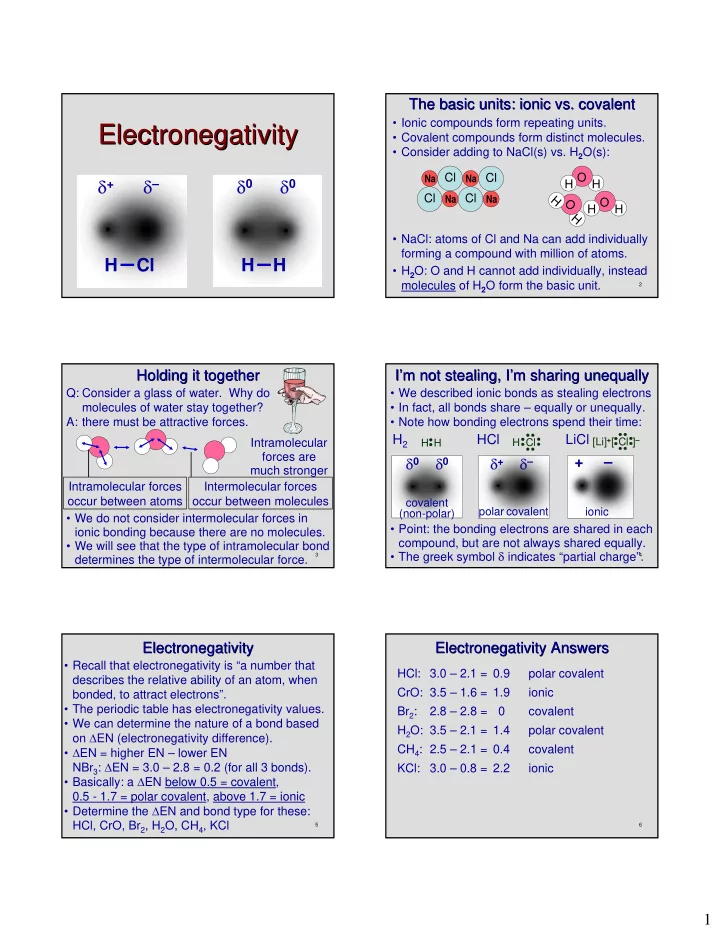

The basic units: ionic vs. covalent The basic units: ionic vs. covalent • Ionic compounds form repeating units. Electronegativity Electronegativity • Covalent compounds form distinct molecules. • Consider adding to NaCl(s) vs. H 2 O(s): Cl Cl O δ + δ – δ 0 δ 0 Na Na H H Cl Na Cl Na H O O H H H • NaCl: atoms of Cl and Na can add individually forming a compound with million of atoms. H Cl H H • H 2 O: O and H cannot add individually, instead molecules of H 2 O form the basic unit. 1 2 Holding it together I’ ’m not stealing, I m not stealing, I’ ’m sharing unequally m sharing unequally Holding it together I Q: Consider a glass of water. Why do • We described ionic bonds as stealing electrons molecules of water stay together? • In fact, all bonds share – equally or unequally. A: there must be attractive forces. • Note how bonding electrons spend their time: H 2 HCl LiCl [Li] + [ Cl ] – Intramolecular H H H Cl forces are δ 0 δ 0 δ + δ – + – much stronger Intramolecular forces Intermolecular forces occur between atoms occur between molecules covalent polar covalent ionic (non-polar) • We do not consider intermolecular forces in • Point: the bonding electrons are shared in each ionic bonding because there are no molecules. compound, but are not always shared equally. • We will see that the type of intramolecular bond • The greek symbol δ indicates “partial charge”. 3 4 determines the type of intermolecular force. Electronegativity Electronegativity Electronegativity Answers Electronegativity Answers • Recall that electronegativity is “a number that HCl: 3.0 – 2.1 = 0.9 polar covalent describes the relative ability of an atom, when CrO: 3.5 – 1.6 = 1.9 ionic bonded, to attract electrons”. • The periodic table has electronegativity values. Br 2 : 2.8 – 2.8 = 0 covalent • We can determine the nature of a bond based H 2 O: 3.5 – 2.1 = 1.4 polar covalent on ∆ EN (electronegativity difference). CH 4 : 2.5 – 2.1 = 0.4 covalent • ∆ EN = higher EN – lower EN NBr 3 : ∆ EN = 3.0 – 2.8 = 0.2 (for all 3 bonds). KCl: 3.0 – 0.8 = 2.2 ionic • Basically: a ∆ EN below 0.5 = covalent, 0.5 - 1.7 = polar covalent, above 1.7 = ionic • Determine the ∆ EN and bond type for these: HCl, CrO, Br 2 , H 2 O, CH 4 , KCl 5 6 1
Electronegativity & physical properties Electronegativity & physical properties CaCl 2 would have a lower Note: other factors • Electronegativity can help to explain properties melting/boiling point: such as atomic size of compounds like those in the lab. δ + δ – CaCl 2 = 3.0 – 1.0 = 2.0 within molecules δ + δ – • Lets look at HCl: partial charges CaF 2 = 4.0 – 1.0 = 3.0 also affects melting δ + δ – δ δ + keep molecules together. LiBr would have a lower and boiling points. δ δ – ∆ EN is an important melting/boiling point: • The situation is similar in NaCl, KCl = 3.0 – 0.8 = 2.2 – factor but not the + but the attraction is even greater LiBr = 2.8 – 1.0 = 1.8 only factor. It is – ( ∆ EN = 2.1 vs. 0.9 for HCl). + most useful when H 2 S would have a lower comparing atoms melting/boiling point: • Which would have a higher melting/boiling point? NaCl because of its greater ∆ EN. and molecules of H 2 O= 3.5 – 2.1 = 1.4 H 2 S = 2.5 – 2.1 = 0.4 similar size. • For each, pick the one with the lower boiling 7 8 point a) CaCl 2 , CaF 2 b) KCl, LiBr c) H 2 O, H 2 S Why oil and water don’ ’t mix t mix Why oil and water don • Lets take a look at why oil and water don’t mix (oil is non-polar, water is polar) δ + δ – δ + δ + δ – δ + Chemical Symbols of δ + δ – δ + δ + δ – δ + Common Elements δ + δ – δ + δ + δ – δ + Prepare a chart in your notes … The partial charges on δ + water attract, pushing δ + δ – δ + δ – δ + the oil (with no partial δ + δ – δ + charge) out of the way. 9 10 Z Name Symbol *Latin *Mnemonic Mnemonics 1 Hydrogen H • Mnemonics are ways to help you remember 2 Helium He • Used by A students and experts • They are often rhymes or visual connections Element Latin name Complete for • E.g. “Thirty days has September, April, June Copper Cuprum elements:1-20, 26, 28- and November, all the rest have 31” Gold Aurum 30, 35, 47, 50, 53, 79, • Or using your knuckles to remember months Iron Ferrum 80, 82, 92 • Iron (Fe) … Lead Plumbum Use the chart on left to • Iron → strong → opposite is feeble → Fe Mercury Hydrargyrum complete the two last • A bad mnemonic for Cu is a copper cup (any columns (only listed Potassium Kalium metal can be made into a cup) elements will have Silver Argentum • A good mnemonic is a cup full of pennies anything for these Sodium Natrium • It may seem like more to know, but it works 11 12 columns). Tin Stannum 2
Best Mnemonics Best Mnemonics Sodium (Na) Iron (Fe) Salt? ↔ Bad for BP ↔ (Na, don’t want it) Ironing with Feet French for iron is Fer North Atlantic salt water Iron = strong = giant = Fe-Fi-Fo-Fum Potassium (K) Copper (Cu) Potatoes covered in ketchup Cu (see you) at copperfields Koala (or kangaroo) eating bananas Cop drinking out of a cup Putting Special K into rolling paper A cup filled with pennies 13 14 Best Mnemonics Best Mnemonics Silver (Ag) Mercury (Hg) Almost gold thermometer = hug to stay warm Aging = grey hair = silver Huge globe (Hg), Hot gas, High gravity Tin (Sn) Lead (Pb) “Tin is Sin” Peanut butter coming out of a pencil Gold (Au) Always united (wedding ring) Pellets and buckshot Gold = shiny = aura Plumber uses lead pipes leaves changing gold colour = Autumn 15 16 For more lessons, visit www.chalkbored.com 3
Recommend
More recommend