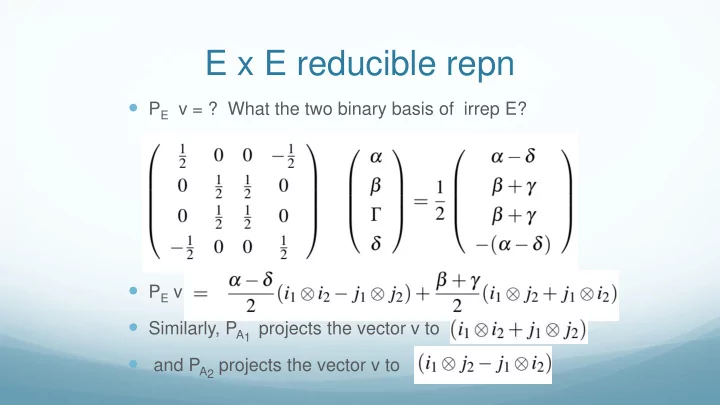

E x E reducible repn P E v = ? What the two binary basis of irrep E? P E v Similarly, P A1 projects the vector v to and P A2 projects the vector v to
Recall Character table Binary basis emerging from tensor product (x,y) component of electric dipole moment p vector belongs to irrep E z-component of p belongs to A 1 All observables can be associated with irreps
Recall Character table Binary basis emerging from tensor product What about quadrupole moment tensor- binary basis
SELECTION RULES For operator f, whether this is non-zero/zero. Gives allowed/forbidden transitions
Selection rules
Selection rules contd For a system with group symmetry G, the transition from an initial state to final state due to interaction is Is this zero or non-zero? Note that initial state belongs to one irrep αof G Final state also to an irrep β of G The interaction operator belongs to an irrep γ The integrand belongs to The transition is allowed if the tensor product allows A 1
Examples Let us look at electric dipole moment transitions for systems with group symmetry C 4v A 1 A 2 Is this allowed or forbidden?
Electric dipole moment belongs to irrep F 1 of O F 1 × A 1 =F 1, F 1 × A 2 =F 2, F 1 × E=F 1 + F 2 F 1 × F 1 =A 1 +E+ F 1 +F 2 , F 1 × F 2 =A 2 +E+ Belongs to F 2 of O F 1 +F 2 , A 1 to A 2 electric dipole moment transition is forbidden.
Polar vector Vs Axial vector Polar vectors are same as axial vectors for molecules with no inversion symmetry/mirror symmetry. For group O, the selection rules is same for electric dipole moment and magnetic dipole moment. For group T d , the selection rule for electric dipole moment transitions is different from the selection rule for magnetic moment transitions. Write the selection rules for electric dipole and magnetic dipole transitions for group D 3d
Molecular vibrations Classical problem of two masses connected by spring Frequency of oscillation/vibration is Where ϰ is stiffness constant and μ is reduced mass of the system As the number of masses in the system increase, the number of degrees of freedom ( dof ) increase and oscillatory motion becomes complicated The number of vibrational degree of freedom is 3N-6- Why? What will be the number of vibrational dof for linear molecule
Molecular vibrations For a complex system with s dof Let (x 1 , x 2, … x s ) denote small excursions of mass points whose Lagrangian is We can diagonalise this so that where η ɭ are the normal coordinates
Vibrations of the non-linear molecule For non-linear triatomic molecule, there will be 3 vibrational modes Bond length changes or bond angle changes
Vibrational modes of nonlinear triatomic molecule
Recommend
More recommend