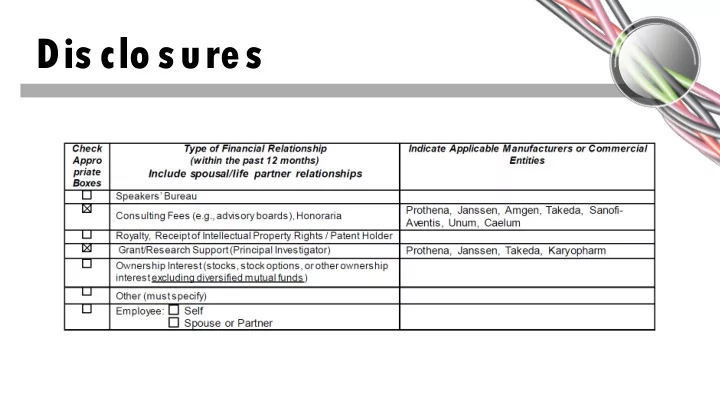

Dis clo sures
O utline • Background • RNAi • Mec hanisms and delivery • ATTR: Clinical results • AL: In vivo models • Monoc lonal antibodies • Spec trum of clinical ac tivity • Anti-amyloid • D ual monoclonal antibody therapy • ATTR: O n-going trial • AL: In vivo model • Conc lusions and Perspec tives
Amyloidosis • Culprit proteins • Transthyretin • Immunoglobulin light chains • Cellular Phase • Production • Trafficking • Sec retion • Extracellular Phase • D issoc iate • Circulate • Misfold • Fibrillate ( β -structure] • Deposit • D isplace
TTR 16 16 to to 40 40 mg mg/dL /dL Re Retinol tinol-bind binding ing pr protei otein n + re + reti tinol nol Th Thyrox oxine ine PNAS 2012;109:9629
FLC In133 AL patients at diagnosis, 84% λ Median edian iFLC FLC 13 135mg/L 5mg/L (2 (29.4 9.4-9780) 9780) Medi edian an dF dFLC LC 12 123m 3mg/L g/L (4 (4-9770) 9770)
RNA Interferenc e • Com omplementary plementary to to ta target rget mRNA NA • Degradat gradation ion of ta f targ rget et mRNA NA -- -- sto topping pping tr translation anslation • An Anti tisen sense se oligo igonu nucleotid cleotides es (AS ASO) O) • Synth ntheti etic c shor ort t sin ingle le-str stran and RNA/DNA hybrid rids s • Modif ifie ied d suga gar groups ups • Secondary condary structure cture and d bin inding ding energy ergy li limi mitations ions • Inhibit ibit mRNA spli licin ing and nd activate ivate RNase e H1 to cle leave ve the e dup uple lex • Small all inte terf rfering ering RNA (siRN iRNA) A) • Shor ort t dsRNA wit ith mo modif ifica icati tions, ons, cle leave ved d by DICER • Activa ivati ting ng the RNA-in induced duced sil ilenc ncing ing compl plex ex (RISC) • Sense strand and becomes s part of RISC • Seeking eking targe get mRNA
RNA Interferenc e • Cha hallen enge ges s of of Effec ecti tive ve Del eliver ery • Prot otec ectio tion n from om ser erum um nu nuclea eases es in c n circul ulat atio ion • Man any veh ehicles: es: virus uses es, , pr prot otei eins, ns, pa particle les • Li Lipi pid d en encap apsul ulat atio ion n (na nano nopa particl icles es) id + and sugar id - back • ion ioniz izabl able e li lipid ar-phos phosphate phate nucleic cleic acid ckbone bone • Hom oming ng to t o tar arge get t cel ells or or or orga gan n syste tem • Clea earan ance ce by by li liver er = Del eliver ery y to o liver er • Ent ntry an and u d unl nloa oadi ding ng of of car argo go • Int ntrac acel ellu lular ar traf affi fickin cking
ATTR Inotersen ( ASO ) • ATTRm / PN • Phase III RCT • 2:1 randomization • 2/2013 - 11/2017 • N = 172 • ~50% V30M • Δ NIS+7 • 2 o cardiac endpoints • 22% withdrawal rate • 3% thrombocytopenia NEJM 2018;379:23
ATTR Patisiran ( siRNA ) • ATTRm / PN • Phase III RCT • 2:1 randomization • 12/2013 - 1/2016 • N = 225 • V30M • 38% / 52% • Δ NIS+7 • 2 o cardiac endpoints • 5% withdrawal rate NEJM 2018;379:11
ATTR Patisiran ( siRNA ) • Prealbumin 25 to 8.9 mg/dL • BNP (pg/mL) • Vitamin A (mcg/dL)
AL TMC 534 ( siRNA ) • κ and λ c onstant region • Preclinical • In vitro • Patient CD138+ cells • NSG mice xenografts • 12 day models • JJN3, MM1.S • IP delivery • Lipidoid nanoparticles • FLC, Flux Blood 2014;123:3440 • sBCMA Gene Ther 2016;23:727 Gene Ther 2019;26:187
Monoclo nal Antibodies • MoA oAb Tar arge gets • Ce Cells lls • Cir Circulating ulating proteins teins • Ebola la virus irus core re receptor ceptor bind inding ing domain main • Co Compl plex exity ity of of de design gn an and de d devel elop opment ent • Hu Human anizati ation on • Hu Human anized ed mou ouse • Mod odificat cations ions an and I d Int ntel ellect lectua ual l Prop oper erty • Ch Chal allen enge ges s of of de deliver ery • Prot otei eins? ns? Agg ggreg egat ates? es? Fibr brils? s? Ch Chap aper eron ones es? Nat Rev 2019;18:585
PRX 004 (anti-TTR) • Anti-TTR monomer • Electrophoresis • Coomassie blue • Partially denatured • Δ Charge • Δ Size • Δ Glycosylation • Phase I clinical trial • CT03336580 PNAS 2012;109:9629 • Accruing BBA 1998;1407:185 • radhika.tripuraneni@prothena.com
CAEL- 101 (anti-amyloid) Blood 2010;116:2241 Blood 2018;132:958 Blood 2018;132:1003
NEO D 001 (anti-amyloid) Stage IV: Cardiac stage 3 dFLC>180mg/L PLOS One 2012 Amyloid 2016 No longer in development Prothena April 18, 2019
D ual MoAb Therapy Ant nti-CD3 CD38, 8, an anti-FLC LC ag aggr greg egat ates es EHA 2019 JCO 2019;37:8009a Cl Lymph Myelom Leuk in press
BCMA-based Therapies for AL Gene Ther 2019;26:187 Poster 4409 – Amandeep Godara, MD Monday, December 9, 2019: 6:00 PM-8:00 PM, Hall B
ALM 1 - If Ebola c an be cle ared . . . • Anti- λ light chain monoclo nal antibody • In vitro activity • SPR, IP/IB • Epitope mapping • Humanization underway • In vivo activity in NSG mouse xenograft model • Testing in vitro against patient samples • Use in MAINTENANCE to achieve iFLC < 10 mg/L ? Poster 4369 – Amandeep Godara, MD Monday, December 9, 2019: 6:00 PM-8:00 PM, Hall B
Conclu sions & Perspec tives • Broad interest in amyloidosis, ATTR and AL (and ALect2 . . .) • Mature and evolving technologies • Next-generation RNAi for ATTR • Monoclonal antibodies against the culprit cells and proteins • Cryo-EM, CRISPR, NGS • Patient advocacy • Amyloidosis Patient Forum with the FDA (https://arci.org) • Support groups around the globe • Investigator and travel grants • Focus in cardiology • American Society of Nuclear Cardiology • Continue to drive for early diagnosis and novel clinical research
Ac knowledgements Demarest Lloyd Jr Foundation Laboratory The Amyloidosis Foundation Ping Zhou Werner and Elaine Dannheiser Fund for Xun Ma Research on the Biology of Aging Adin Kugelmass of the Lymphoma Foundation The Amyloidosis and Myeloma Research Fund Denis Toskic MMRF Amandeep Godara The Cam Neely and John Davis Myeloma Research Fund Janssen Neely Center for Clinical Cancer Research Cindy Varga, MD Terry Fogaren, NP
Recommend
More recommend