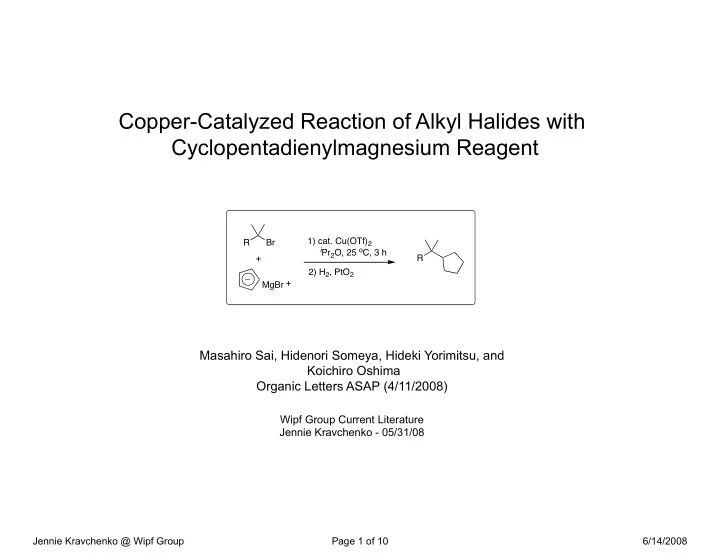

Copper-Catalyzed Reaction of Alkyl Halides with Cyclopentadienylmagnesium Reagent 1) cat. Cu(OTf) 2 R Br i Pr 2 O, 25 o C, 3 h + R 2) H 2 , PtO 2 + MgBr Masahiro Sai, Hidenori Someya, Hideki Yorimitsu, and Koichiro Oshima Organic Letters ASAP (4/11/2008) Wipf Group Current Literature Jennie Kravchenko - 05/31/08 Jennie Kravchenko @ Wipf Group Page 1 of 10 6/14/2008
Copper-Catalyzed Cross-Coupling Reactions of Grignard Reagents with Primary Alkyl Halides Alkyl Bromides: RMgCl Tetrahedron 2000 , 56, 2737 Br R cat. Li 2 CuCl 4 , 20 o C Alkyl Fluorides: cat. CuCl 2 J. Am. Chem. Soc. 2003 , 125, 5646 + R F R' MgX R R' 1,3-butadiene R = alkyl R' = alkyl, aryl Alkyl Chlorides: cat. CuCl 2 Angew. Chem. Int. Ed. 2007 , 46, 2086 cat. Ph Me + Alkyl Cl R MgX Alkyl R THF Jennie Kravchenko @ Wipf Group Page 2 of 10 6/14/2008
Cobalt-Catalyzed Cross-Coupling Reactions of Grignard Reagents with Secondary and Tertiary Alkyl Halides Substrate Scope: cat. [CoCl 2 (dppp)] O n -C 4 H 9 O O O H 2 C CHCH 2 MgCl cat. [CoCl 2 (dppp)] I THF, - 40 o C H 2 C CHCH 2 MgCl R X CH 3 then CrO 3 /acetone R THF, temp. 1 2 1 Temperature ( o C) Yield of 2 (%) H 3 C C 6 H 11 - 20 83 O O Ph Br n -C 4 H 9 O n -C 4 H 9 O CH 3 t -C 4 H 9 0 76 Br - [CoCl 2 (dppp)] effectively catalyzes such reactions of secondary and tertiary alkyl halides with allylic 20 31 n -C 8 H 17 Cl Grignard reagents n -C 6 H 13 - Treatment of substrate with allyl Grignard reagent O in the presence of [CoCl 2 (dppp)] furnished the ring - 40 76 -opening product I - Existence of radical intermediates account for such ring opening Angew. Chem. Int. Ed. 2002 , 41, 4137 Jennie Kravchenko @ Wipf Group Page 3 of 10 6/14/2008
Cobalt-Catalyzed Cross-Coupling Reactions of Grignard Reagents with Secondary and Tertiary Alkyl Halides Proposed Mechanism: 1 2 3 R R X R Co R 1) Single-electron transfer from cobalt complex 2) Recombination of alkyl radical and cobalt complex 3) Reductive elimination - π -Allyl ligands may prevent the formation of the vacant coordination sites necessary for ß-elimination, which enables allylation of tertiary and secondary alkyl halides as well as of alkyl halides having ß-alkoxy groups Angew. Chem. Int. Ed. 2002 , 41, 4137 Jennie Kravchenko @ Wipf Group Page 4 of 10 6/14/2008
Silver-Catalyzed Cross-Coupling Reactions of Grignard Reagents with Secondary and Tertiary Alkyl Halides Substrate Scope: R R cat. AgNO 3 cat. AgNO 3 Alkyl X + BrMg Alkyl Alkyl X + BrMg Alkyl Et 2 O, 25 o C, 3 h Et 2 O, 25 o C, 3 h 1 2 1 2 1 cat./mol% Grignard reagent Yield of 2 (%) 1 cat./mol % Yield of 2 (%) 1.0 R = H (1.3 equiv) 83 1.0 87 n -C 8 H 17 Br n -C 8 H 17 Br 1.0 66 2.5 R = H (1.3 equiv) 80 n -C 8 H 17 Cl n -C 6 H 13 Br 1.0 14 I 1.0 R = Me (1.5 equiv) 80 n -C 8 H 17 Br CH 3 1.0 81 t -C 4 H 9 Br 2.5 R = Me (1.5 equiv) 79 n -C 6 H 13 Br - Silver-catalyzed conditions were applicable to benzylation, as well as allylation and methallylation of secondary and tertiary alkyl halides - Analogous radical pathway was proposed Organic Letters 2008 , 10, 971 Jennie Kravchenko @ Wipf Group Page 5 of 10 6/14/2008
Copper-Catalyzed Cyclopentadienylation of Secondary and Tertiary Alkyl Halides Followed by Hydrogenation General Reaction: 1a n -C 8 H 17 Br 5 mol % Cu(OTf) 2 n -C 8 H 17 + i- Pr 2 O, 25 o C, 3 h 2a MgBr + 10 mol % PtO 2 , 0.1 MPa H 2 n -C 8 H 17 n -C 8 H 17 n -C 8 H 17 + AcOH, reflux, 12 h 4a 3a 3a' 85% overall yield 95% combined yield - Initially formed 2a undergoes isomerization into 3a and 3a’ due to the high acidity of the hydrogen on the cyclopentadienyl ring - Isomers were subjected to hydrogentation in order to simplify analysis of products Organic Letters ASAP (4/11/2008) Jennie Kravchenko @ Wipf Group Page 6 of 10 6/14/2008
Solvent Effect and Catalyst Screening: - Choice of solvent and copper catalyst n -C 8 H 17 n -C 8 H 17 Br greatly altered overall yield of products 5 mol % catalyst + + solvent, 25 o C, 3 h - Bulky ethers (diisopropyl ether and t -butyl methyl ether) proved to be most MgBr + n -C 8 H 17 suitable - Copper(II) halides as well as copper(I) entry solvent catalyst combined yield (%) halides exhibited modest catalytic 1 i -Pr 2 O Cu(OTf) 2 96 activity 2 t - -BuOMe Cu(OTf) 2 68 3 toluene Cu(OTf) 2 90 - Silver(I) nitrate, found to be effective in 4 diethyl ether Cu(OTf) 2 16 the cross-coupling reaction of tertiary 5 dioxane Cu(OTf) 2 12 6 THF Cu(OTf) 2 15 alkyl halides with allyl or benzyl 7 c -C 5 H 11 OMe Cu(OTf) 2 13 Grignard reagents, was less effective 8 Bu 2 O Cu(OTf) 2 22 than copper(II) triflate 9 i -Pr 2 O CuF 2 77 10 i -Pr 2 O CuCl 2 59 11 i -Pr 2 O CuCl 44 12 i -Pr 2 O CuBr 57 13 i -Pr 2 O CuI 31 14 i -Pr 2 O CuOAc 30 15 i -Pr 2 O CuCN 26 16 i -Pr 2 O CuOTf 0.5 C 6 H 6 27 17 i -Pr 2 O AgNO 3 26 Organic Letters ASAP (4/11/2008) Jennie Kravchenko @ Wipf Group Page 7 of 10 6/14/2008
Substrate Scope: Alkyl X 1 Alkyl Alkyl 5 mol % Cu(OTf) 2 + + i- Pr 2 O, 25 o C, 3 h 3 3' - Phenylsulfanyl and methoxyl MgBr + groups were compatible in such Alkyl 10 mol % PtO 2 , 0.1 MPa H 2 reaction conditions without deactivation of the copper catalyst AcOH, reflux, 12 h 4 - Surprisingly, tertiary alkyl fluoride combined yield overall yield alkyl-X of 3 and 3' (%) of 4 from 1 (%) participated in cyclopentadienylation as well 88 80 n -C 8 H 17 Cl 90 84 MeO Br 5 95 50 PhS Br 5 51 49 n -C 6 H 13 Br 69 61 Ph F 2 Organic Letters ASAP (4/11/2008) Jennie Kravchenko @ Wipf Group Page 8 of 10 6/14/2008
Stoichiometric Reactions: n -C 8 H 17 Br n -C 8 H 17 - Reaction mechanism was studied using (0.50 mmol) 1.0 equiv Cu(OTf) 2 the following halide with stoichiometric + + copper reagents and varying amounts of i -Pr 2 O, 25 o C, 3 h CpMgBr n -C 8 H 17 + - Copper reagent that is active for this MgBr reaction might be [Cp 3 Cu]MgBr 6 or a more complex cuprate - Experiments have been conducted to amount of examine the intermediacy of alkyl Grignard reagent NMR yield radicals in the reaction, however they 1.0 equiv 0% failed to support the intermediacy 2.0 equiv 15% 3.0 equiv 86% Organic Letters ASAP (4/11/2008) Jennie Kravchenko @ Wipf Group Page 9 of 10 6/14/2008
Conclusions and Future Directions - Copper(II) triflate proved to efficiently catalyze the reaction of tertiary alkyl halides with cyclopentadienyl Grignard reagent. - With the following hydrogenation of the cyclopentadienyl ring with hydrogen under Pt 2 O catalysis, the overall transformation represents formal cyclopentylation of tertiary alkyl halides. - Future work could include extension of the methodology to other stabilized organometallic reagents, as well as application of this method to functionalized molecules of interest. Jennie Kravchenko @ Wipf Group Page 10 of 10 6/14/2008
Recommend
More recommend