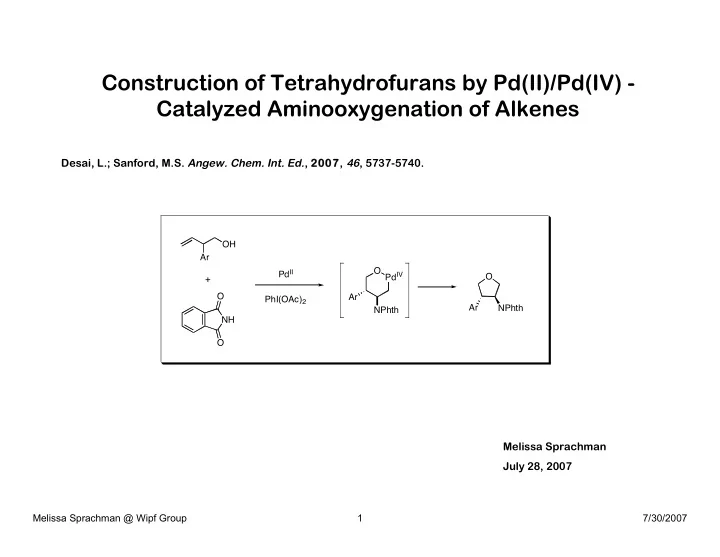

Construction of Tetrahydrofurans by Pd(II)/Pd(IV) - Catalyzed Aminooxygenation of Alkenes Desai, L.; Sanford, M.S. Angew. Chem. Int. Ed. , 2007, 46 , 5737-5740. OH Ar O Pd IV Pd II O + O Ar PhI(OAc) 2 Ar NPhth NPhth NH O Melissa Sprachman July 28, 2007 Melissa Sprachman @ Wipf Group 1 7/30/2007
Oxidative Functionalization Via Pd IV Intermediates PhI(OAc) 2 + Pd IV (OAc) 3 Pd(OAc) 2 OAc -AcOH PhH + Pd(OAc) 2 Pd II (OAc) Pd II + Pd(0) PhH Yoneyama, T.; Crabtree, R.H. J. Mol. Catal. A 1996 , 108 , 35. Slide info first found in: Gorin, D. ACS Fellowship essay, Univ. California Berkeley, Berkeley, CA 2006. Dick, A.R.; Hull, K.L.; Sanford, M.S. J. Am. Chem. Soc. 2004 , 126 , 2300. Melissa Sprachman @ Wipf Group 2 7/30/2007
Studies on Isolable Pd IV Complexes Synthesis of Stable Pd IV Complexes: R = H, Me; X = R, Ar, X, NO 2 C-O Bond Forming Reductive Elimination Upon Heating: Dick, A.R.; Kampf, J. W..; Sanford, M.S. J. Am. Chem. Soc. 2005 , 127 , 12790. Melissa Sprachman @ Wipf Group 3 7/30/2007
Aminopalladation Background R 2 N H -HPdX X R 2 N -HX R Pd NH X R Pd --Aliphatic amines often displace the alkene from the palladium center --Amides are viable alternatives due to their decreased basicity. O 5 % PdCl 2 (MeCN) 2 O Z 5 % CuCl Z + + Pd(II) NH N Pd NH 2 NH 2 1 atm O 2 , DME (n) (n) Z = EWG + Pd(II) NTs NHTs Hegedus, L.S. Tetrahedron, 1984, 40 , 2415. Hosokawa, T. In Handbook of Organopalladium Chemistry for Organic Synthesis : Negishi, E.-I., Ed.; John Wiley and Sons: New York, 2002, vol 2, pp 2211-2213. Melissa Sprachman @ Wipf Group 4 7/30/2007
Recent Work in Aminopalladation/Aminoacetoxylation Chemistry O O O 10 mol% Pd(OAc) 2 10 mol% Pd(OAc) 2 2 eq PhI(OAc) 2 2 eq PhI(OAc) 2 TsN TsN O O TsHN O O 1 equiv Bu 4 NOAc 1 equiv Bu 4 NOAc TsHN O CH 3 CN,60 o C, 2.5 h CH 3 CN, 25 o C, 7 h OAc OAc 65%, >20:1 dr 92%, 9.5:1 dr Alexanian, E. J.; Chulbom, L.; Sorenson, E.J. J. Am. Chem. Soc. 2005, 127 , 7690. Melissa Sprachman @ Wipf Group 5 7/30/2007
Stahl and Sanford’s Approach to Aminoacetoxylation � -Hydride Eliminaton (Pd 0 /Pd II Manifold) -[L n Pd ii H] NPhth C 6 H 13 C 6 H 13 + O 5 mol % Pd(OAc) 2 NH O CH 2 Cl 2 , 60 o C O N [L n Pd II ] O (Aminopalladation) C 6 H 13 2 equiv PhI(OAc) 2 NPhth NPhth via Pd IV AcO C 6 H 13 AcO C 6 H 13 -[L n Pd II ] Oxidative Functionalization (Pd II /Pd IV Manifold) Melissa Sprachman @ Wipf Group 6 7/30/2007
Intermolecular Pd-Catalyzed Aminoacetoxylation of Alkenes (Stahl) O PdCl 2 (CH 3 CN) 2 NPhth NPhth OAc 2.5 equiv PhI(OAc) 2 + + + OAc OAc C 6 H 13 NH C 6 H 13 C 6 H 13 C 6 H 13 O 60 : 19 : 28 Key Features: --Excellent Regioselectivity (no phthalimide addn at the terminal carbon observed) --Substantial yields, even for unactivated alkenes Yield Product 1 H NMR (isolated) Alkene -- Single diastereomers observed by 1 HNMR: --Favorable yields with allylic Oxygens suggest chelation; Yields drop with vinyl ethers (45%) And homoallylic ethers (30%) Liu, G.; Stahl, S. J. Am. Chem. Soc. 2006, 128 , 7179. Reactions with PdCl 2 (CH 3 CN) 2 and PhI(OAc) 2 Melissa Sprachman @ Wipf Group 7 7/30/2007
Sanford: Suppression of � -Hydride Elimination Using Tethered Alcohols O O + NPhth OAc OH 45% NPhth 10 mol % Pd(OAc) 2 3 equiv PhI(OAc) 2 + 20 mol% AgBF 4 O [Pd] CH 3 CN, 60 o C O NH H NPhth NPhth O or AcO OH OH --Coordination of the alcoholic oxygen to Pd slows � -hydride elimination Possible S N 2 Mechanisms for Aminooxygenation: O O O NPhth S N 2 S N 2 + NH AcO OH NPhth O 2 CPh O --Subjection of authentic samples of either substrate did not yield amidated THF products Melissa Sprachman @ Wipf Group 8 7/30/2007
Alternative Routes Considered O Direct Red. Elim. O Ph OH L n Pd IV NPhth Ph PhthN Ph [L n Pd II ] + cis Aminopalladation/oxidation PhI(OAc) 2 O NH NPhth O S N 2 Ph L n Pd IV O OH Ph PhthN NPhth S N 2 O Ph L n Pd IV OH OH Ph Ph PhthN [L n Pd II ] + trans Aminopalladation/oxidation PhI(OAc) 2 O NH Direct Red. O O Ph Elim. O L n Pd IV NPhth PhthN Ph Only the trans THF products were observed. Melissa Sprachman @ Wipf Group 9 7/30/2007
Determination of the Stereochemistry of Aminopalladation OH Ph 5 mol % Pd(OAc) 2 NPhth NPhth 10 mol % AgBF 4 + + Ph OH OH CH 3 CN, 60 o C, O 2 Ph O 3% NH > 10 : 1 O From cis aminopalladation Liu, G.; Stahl, S. J. Am. Chem. Soc. 2006, 128 , 7179. Support from Stahl’s Work: OAc 10 mol% [Pd] NPhth + PhthNH + Ph OMe Ph OMe Ph OMe 2.5 eq. PhI(OAc) 2 NPhth DCE, 70 o C, 20 h : 52% [Pd] = PdCl 2 (CH 3 CN) 2 5% Pd(OAc) 2 : 44% 0% Possible routes: A) trans -aminopalladation followed by oxidative Pd-C cleavage with retention B) cis -aminopalladation followed by oxidative Pd-C cleavage with inversion Melissa Sprachman @ Wipf Group 10 7/30/2007
Decreased diastereoselectivities were observed with substituents in the meta and para positions Melissa Sprachman @ Wipf Group 11 7/30/2007
Conclusions -3 aminotetrahydofurans were unexpectedly synthesized in Sanford and coworkers’ attempts to suppress the beta-hydride elimination pathway of an aminopalladation/oxidation sequence. -Evidence from experiments by Stahl and Sanford supports cis -aminopalladation of alkenes. -The promising diastereoselectivities and consequent mechanistic elucidation may lay the framework for the development of enantioselective Pd II /Pd IV reactions. Melissa Sprachman @ Wipf Group 12 7/30/2007
Recommend
More recommend