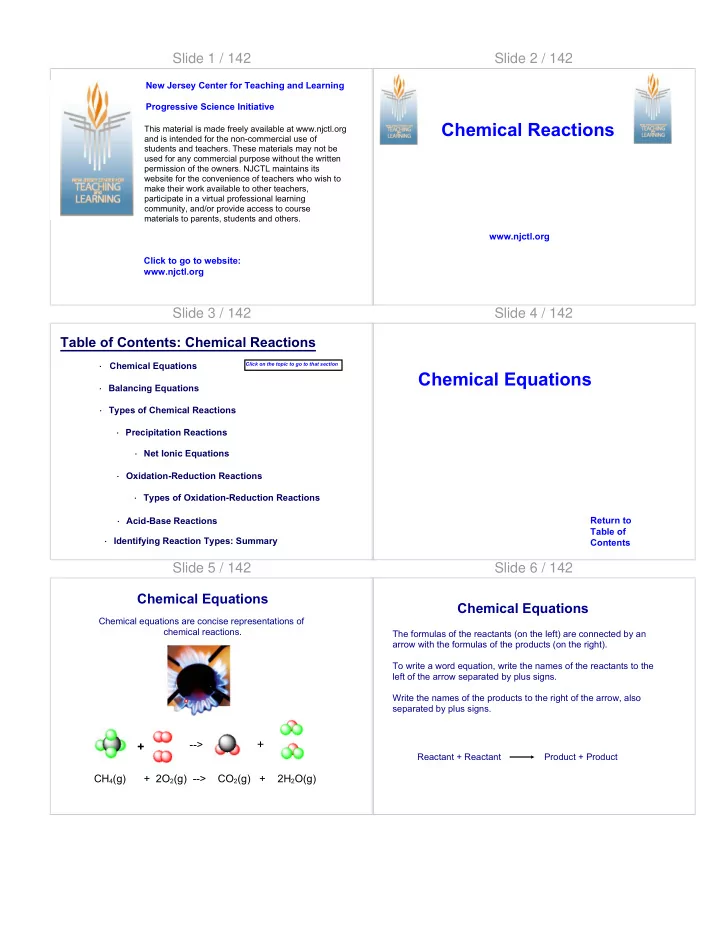

Slide 1 / 142 Slide 2 / 142 New Jersey Center for Teaching and Learning Progressive Science Initiative Chemical Reactions This material is made freely available at www.njctl.org and is intended for the non-commercial use of students and teachers. These materials may not be used for any commercial purpose without the written permission of the owners. NJCTL maintains its website for the convenience of teachers who wish to make their work available to other teachers, participate in a virtual professional learning community, and/or provide access to course materials to parents, students and others. www.njctl.org Click to go to website: www.njctl.org Slide 3 / 142 Slide 4 / 142 Table of Contents: Chemical Reactions Chemical Equations Click on the topic to go to that section · Chemical Equations · Balancing Equations · Types of Chemical Reactions · Precipitation Reactions · Net Ionic Equations · Oxidation-Reduction Reactions · Types of Oxidation-Reduction Reactions Return to · Acid-Base Reactions Table of · Identifying Reaction Types: Summary Contents Slide 5 / 142 Slide 6 / 142 Chemical Equations Chemical Equations Chemical equations are concise representations of chemical reactions. The formulas of the reactants (on the left) are connected by an arrow with the formulas of the products (on the right). To write a word equation, write the names of the reactants to the left of the arrow separated by plus signs. Write the names of the products to the right of the arrow, also separated by plus signs. ⇒ + --> + Reactant + Reactant Product + Product CH 4 (g) + 2O 2 (g) --> CO 2 (g) + 2H 2 O(g)
Slide 7 / 142 Slide 8 / 142 Symbols used in chemical equations Skeleton equations A skeleton equation is a chemical equation that does not indicate the relative amounts of the reactants and products. Write the formulas of the reactants to the left of the yields sign (arrow) and the formulas of the products to the right. Here is the equation for rusting: Metallic Iron reacts with oxygen in the air to produce iron (III) oxide (rust). Iron ( metal) + Oxygen ( gas) ⇒ iron (III) oxide ( solid) (word equation) Fe + O 2 Fe 2 O 3 ( skeleton /chemical equation) Slide 9 / 142 Slide 10 / 142 1 In the reaction CH 4 ( g ) + O 2 ( g ) H 2 O ( g ) + CO 2 ( g ) Word Equations the products are: When ignited, methane gas reacts with oxygen gas A oxygen and water to produce carbon dioxide and steam. B carbon dioxide and water ⇒ + + C oxygen and methane D methane and carbon dioxide CH 4 gas CO 2 gas O 2 gas H 2 O gas E I don't know the answer to this. answer This "skeleton" equation is not balanced: CH 4 ( g ) + O 2 ( g ) H 2 O ( g ) + CO 2 ( g ) Slide 11 / 142 Slide 12 / 142 2 In the reaction CH 4 ( g ) + O 2 ( g ) H 2 O ( g ) + CO 2 ( g ) Word equations to Chemical equations the products are: A solids Solid potasium chlorate decomposes in air to produce solid potassium chloride and oxygen gas. B liquids The word equation is: C gases potasium chlorate (s) --> potassium chloride (s) + oxygen (g) D dissolved in water (aqueous) E cannot be determined The unbalanced "skeleton" equation is: answer F I don't know how to answer this. KClO 3(s) KCl (s) + O 2(g)
Slide 13 / 142 Slide 14 / 142 Law of Conservation of Mass Word equations to Chemical equations Write the word equation, then the skeleton equation Aluminum sulfate reacts with calcium chloride to “We may lay it down as an incontestable axiom produce calcium sulfate and aluminum chloride that, in all the operations of art and nature, nothing is created; an equal amount of matter exists both before and after the experiment. Aluminum sulfate + calcium chloride --> calcium sulfate + aluminum chloride Slide for Word equation Upon this principle, the whole art of performing chemical experiments depends.” --Antoine Lavoisier, 1789 Slide for Skeleton equation Al 2 (SO 4 ) 3 + CaCl 2 --> Ca(SO 4 ) + AlCl 3 Slide 15 / 142 Slide 16 / 142 Balancing chemical equations Balancing Equations To write a balanced chemical equation, first write the skeleton equation. Then use coefficients to balance the equation so that it obeys the law of conservation of mass. This is a balanced equation for making a bicycle. The numbers are called coefficients—small whole numbers that are placed in front of the formulas in an equation in order to balance it. Return to Table of Contents Slide 17 / 142 Slide 18 / 142 Balancing chemical equations Balancing chemical equations CH 4 ( g ) + 2 O 2 ( g ) CO 2 ( g ) + 2 H 2 O ( g ) CH 4 ( g ) + 2 O 2 ( g ) CO 2 ( g ) + 2 H 2 O ( g ) 2 O 1C 1C 4 O 4H 2 O 4H 2 O 1C 1C 4 O 4H 4H 2 O Products appear on the Reactants appear on the right side of the equation. Coefficients are inserted to balance the equation. left side of the equation. The states of the reactants and products are written in parentheses to the right of each compound.
Slide 19 / 142 Slide 20 / 142 Subscripts and Coefficients 3 How many oxygen atoms are in one formula unit of calcium nitrate? (First, write the formula for calcium nitrate.) A 2 B 3 answer C 5 D 6 Subscripts tell the number of atoms of each element in a molecule. E I don't know how to answer this. Coefficients tell the number of representative particles (atoms, molecules, or formula units). Slide 21 / 142 Slide 22 / 142 Balancing chemical equations 4 How many nitrogen atoms are in one formula unit of ammonium sulfate? H 2 + Cl 2 HCl H 2 + Cl 2 2HCl unbalanced balanced answer 2 2 1 2 2 2 2 1 Slide 23 / 142 Slide 24 / 142 Balancing chemical equations Balancing chemical equations Write a balanced chemical equation for this reaction. Then, count up the number of each type of element on each side of the reaction First write a skeleton equation Cl 2 + NaBr Br 2 + NaCl chlorine + sodium bromide bromine + sodium chloride Reactants Products Cl: 2 Cl: 1 Cl 2 + NaBr Br 2 + NaCl Na: 1 Na: 1 Br: 1 Br: 2
Slide 25 / 142 Slide 26 / 142 Balancing chemical equations Balancing chemical equations Next, identify one element that is not balanced. It is best to start with an easy element. The fewer places an element appears on Identify the side that needs more of that particular element. both sides of a reaction, the easier it will be to balance. Cl 2 + NaBr Br 2 + NaCl Cl 2 + NaBr Br 2 + NaCl Reactants Products Reactants Products Cl: 2 Cl: 1 Cl: 2 Cl: 1 Na: 1 Na: 1 Na: 1 Na: 1 Br: 1 Br: 2 Br: 1 Br: 2 Slide 27 / 142 Slide 28 / 142 Balancing chemical equations Balancing chemical equations Determine which molecule or element will be getting the coefficient. In this case, because we need more chlorine on the To figure out what the coefficient should be, simply take the amount of products side, we will have to add a coefficent to the NaCl, since that specific element you need from the molecule, and divide by the that is the only product containing chlorine. amount of the element you have in the molecule. Cl 2 + NaBr Br 2 + NaCl 2 Cl 2 + NaBr Br 2 + __NaCl Reactants Products Reactants Products Cl: 2 Cl: 2 Have = 2 Need Cl: 2 Cl: 1 Na: 1 1 = Cl: 2 Cl: 1 Na: 1 Na: 1 Na: 1 Br: 1 Na: 1 Na: 1 Br: 1 2 Br: 1 Br: 2 Br: 1 Br: 2 If this is not a whole number, simply multiply ALL the substances in the reaction by some whole number to make the coefficients whole numbers. Slide 29 / 142 Slide 30 / 142 Balancing chemical equations Balancing chemical equations Continue with these steps until all the elements are balanced. Now, just reevaluate the amount of each element on the table When all the elements exist in equal amounts on both sides of the equation, you have a balanced chemical equation. Cl 2 + NaBr Br 2 + 2NaCl Cl 2 + 2NaBr Br 2 + 2NaCl Reactants Products Cl: 2 Reactants Products Cl: 2 Cl: 2 Cl: 1 2 Na: 1 Cl: 2 Cl: 1 2 Na: 1 Na: 1 Na: 1 2 Br: 1 Na: 1 2 Na: 1 2 Br: 1 Br: 1 Br: 2 Br: 1 2 Br: 2
Recommend
More recommend