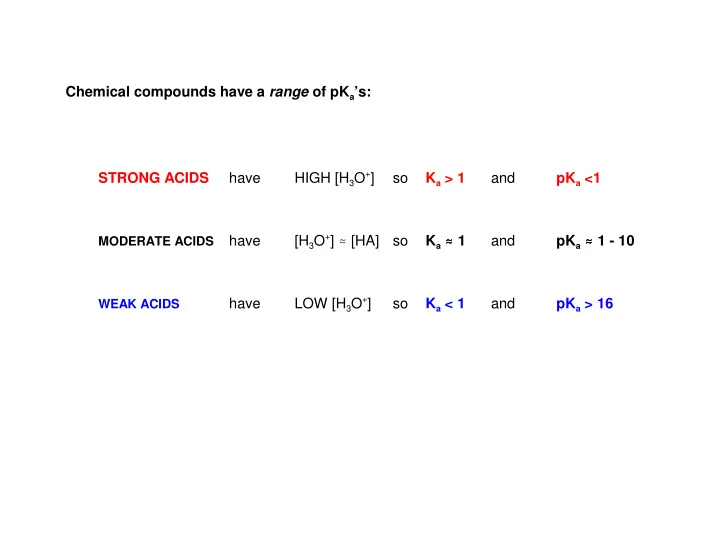

Chemical compounds have a range of pK a ’s: HIGH [H 3 O + ] STRONG ACIDS have so K a > 1 and pK a <1 [H 3 O + ] . [HA] K a . . 1 . . pK a . . . 1 - 10 . have so and MODERATE ACIDS LOW [H 3 O + ] have so K a < 1 and pK a > 16 WEAK ACIDS
Molecular structure and acid strength / base strength 1. Periodic trends in any row of the periodic table, relative acidity of a H–Z bond increases from left to right: acid: H–CH 3 H–NH 2 H–OH H–F pK a : ~51 38 16 3.5 consequently, the relative stability of the conjugate base increases from left to right: - CH 3 - NH 2 - OH - F conjugate base: in any column of the periodic table, relative acidity of a H–Z bond increases from top to bottom: consequently, bigger conjugate bases are more stable conjugate bases: conjugate base: acid: pK a : - F H–F 3.5 - Cl H–Cl -7 - Br H–Br -8 - I H–I -10
Molecular structure and acid strength / base strength relative acidity of a H–Z bond is a function of the hybridization state of Z: C sp 3 –H C sp 2 –H C sp –H H H H H C C C C H C C H H H H H H H pK a : ~50 ~33 ~25 consequently, conjugate bases that have increased s character in the hybridization state of the atom bearing negative charge are more stable: H H .. .. - - . - C C . C C H C C H H H H H H
Molecular structure and acid strength / base strength relative acidity of a H–Z bond is a function of formal positive charge on Z: caused by resonance: - - .. : .. : .. .. .. .. : : O O O O + H + .. H . H - . . O O : O O .. .. . .. + .. pK a = 4.8 .. - H + H + : O O .. .. .. pK a = 16 consequently, resonance-stabilized conjugate bases are weaker bases .
Molecular structure and acid strength / base strength relative acidity of a H–Z bond is a function of electron deficiency on Z: caused by induction: .. :- H + H+ O O .. .. .. pK a = 16 .. F :- F H + H+ O O .. .. .. F F F F pK a = 12.4 consequently, inductively withdrawing functional groups decrease electron density on the atom of the conjugate base bearing negative charge and stabilize it.
Predicting acid-base equilibria 1. Identify the acid and base on the left side of the equilibrium arrow. 2. Identify the conjugate acid and conjugate base on the right side of the equilibrium arrow. 3. Compare the pk a values of the acid and conjugate acid. 4. If there is no table of values, estimate them by judging the relative stabilites of the conjugate bases of the equilibrium. 4. Label the acids strong and weak. 5. Do the same for the base and conjugate base. 6. Strong goes to weak, telling you the direction of the equilibrium. | ∆ pK a | $ 3 means reaction is essentially complete (equilibrium lies fully to the right) or there is 7. essentially no reaction (equilibrium lies fully to the left).
Recommend
More recommend