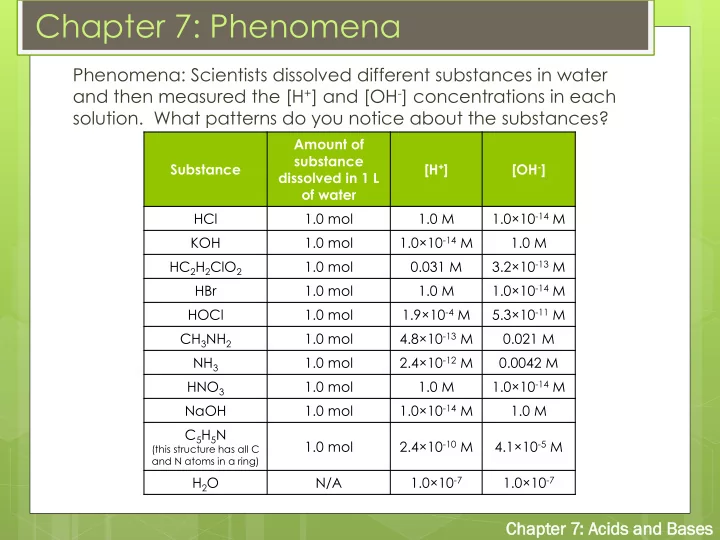

Chapter 7: Phenomena Phenomena: Scientists dissolved different substances in water and then measured the [H + ] and [OH - ] concentrations in each solution. What patterns do you notice about the substances? Amount of substance Substance [H + ] [OH - ] dissolved in 1 L of water 1.0×10 -14 M HCl 1.0 mol 1.0 M 1.0×10 -14 M KOH 1.0 mol 1.0 M 3.2×10 -13 M HC 2 H 2 ClO 2 1.0 mol 0.031 M 1.0×10 -14 M HBr 1.0 mol 1.0 M 1.9×10 -4 M 5.3×10 -11 M HOCl 1.0 mol 4.8×10 -13 M CH 3 NH 2 1.0 mol 0.021 M 2.4×10 -12 M NH 3 1.0 mol 0.0042 M 1.0×10 -14 M HNO 3 1.0 mol 1.0 M 1.0×10 -14 M NaOH 1.0 mol 1.0 M C 5 H 5 N 2.4×10 -10 M 4.1×10 -5 M 1.0 mol (this structure has all C and N atoms in a ring) H 2 O N/A 1.0×10 -7 1.0×10 -7 Cha hapt pter er 7: Acid ids s and Ba Bases ses
Chapter 7 Acids and Big Idea: A Bronsted-Lowry acid is Bases a proton donor and a Bronsted-Lowry base is a proton acceptor. After an acid/base • Acids and Bases loses/gains its proton it • Conjugate Acids/Bases becomes a conjugate • Strength of Acids/Bases • pH/pOH Scales base/acid. Acids and • pH/pOH of Strong bases can either Acids/Bases completely dissociate • pH/pOH of Weak (strong) or incompletely Acids/Bases dissociate (weak). An • Acid/Base Properties of equilibrium problem Salts must be set up to solve • Acid Rain for the pH of a weak acid or base. 2
Acids and Bases Acid Sour taste (lemon citric acid) Dissolve many metals (Acid(aq) + metal(s) → salt(aq) + H 2 (g)) Turn litmus paper red Base Bitter taste (unsweetened baker’s chocolate) Slippery feel (cleaning products) Turn litmus paper blue Cha hapt pter er 7: Acid ids s and Ba Bases ses 3
Acids and Bases Arrhenius (1884) Acid: A compound that forms hydrogen ions (H + ) in water. Examples: • HCl(aq) acid • CH 4 (aq) not an acid because it does not release (H + ) ions in solution Base: A compound that forms hydroxide ions (OH - ) in water. Examples: • NaOH(aq) base • NH 3 base because NH 3 (aq) + H 2 O(l) → NH 4 + (aq) + OH - (aq) Bronsted-Lowry (1923) Acid: A proton donor. Base: A proton acceptor. Cha hapt pter er 7: Acid ids s and Ba Bases ses 4
Acids and Bases Deprotonation: The loss of a proton from a Bronsted-Lowry acid Not ote: e: First deprontination is the loss of the first H, the second deprotination is the loss of a second H, and the third deprotination is the loss of a third H. Amphoteric: A substance that can act as an acid or base Example: • H 2 O Cha hapt pter er 7: Acid ids s and Ba Bases ses 5
Conjugate Acids/Bases Conjugate Acid Base Pair: Two substances that are related to each other by the transfer of one proton 𝑒𝑝𝑜𝑏𝑢𝑓𝑡 𝑏 𝑞𝑠𝑝𝑢𝑝𝑜 𝐷𝑝𝑜𝑘𝑣𝑏𝑢𝑓 𝐶𝑏𝑡𝑓 𝐵𝑑𝑗𝑒 𝑏𝑑𝑑𝑓𝑞𝑢𝑡 𝑏 𝑞𝑠𝑝𝑢𝑝𝑜 𝐷𝑝𝑜𝑘𝑣𝑏𝑢𝑓 𝐵𝑑𝑗𝑒 𝐶𝑏𝑡𝑓 Cha hapt pter er 7: Acid ids s and Ba Bases ses 6
Conjugate Acids/Bases Student Question Which of the following represent conjugate acid-base pairs? For those pairs that are not conjugates, write the correct conjugate acid or base for each species in the pair. a) H 2 SO 4 and SO 4 2- - and HPO 4 b) H 2 PO 4 2- c) HClO 4 and Cl - + and NH 2 d) NH 4 - Cha hapt pter er 7: Acid ids s and Ba Bases ses 7
Strength of Acids/Bases Strong Acid or Base: An acid/base that completely ionizes in solution. Weak Acid or Base: An acid/base that does not completely ionize in solution. Strong Acids Strong Bases HCl HNO 3 LiOH Sr(OH) 2 HBr HClO 4 NaOH Ca(OH) 2 HI HClO 3 KOH Ba(OH) 2 HBrO 3 HBrO 4 RbOH Mg(OH) 2 H 2 SO 4 HIO 4 CsOH Cha hapt pter er 7: Acid ids s and Ba Bases ses 8
Strength of Acids/Bases Name Formula K a Hydrogen Sulfate Ion HSO 4 1.2 × 10 -2 - Chlorous Acid HClO 2 1.2 × 10 -2 Monochloracetic Acid HC 2 H 2 ClO 2 1.35 × 10 -3 Hydrofluoric Acid HF 7.2 × 10 -4 Nitrous Acid HNO 2 4.0 × 10 -4 Acetic Acid HC 2 H 3 O 2 1.8 × 10 -5 Hydrated Aluminum(III) Ion [Al(H 2 O) 6 ] 3+ 1.4 × 10 -5 Hypochlorous Acid HOCl 3.5 × 10 -8 Hydrocyanic Acid HCN 6.2 × 10 -10 Ammonium Ion NH 4 5.6 × 10 -10 + Phenol HOC 6 H 5 1.6 × 10 -10 Not ote: e: A strong acid is defined as an acid that has a K a larger than 1. Not all strong acids have the same K a . For example the K a of H 3 O + is 55, whereas the K a of HCl is 1×10 6 . Therefore, H 3 O + is one of the weakest strong acids. Cha hapt pter er 7: Acid ids s and Ba Bases ses 9
Strength of Acids/Bases Name Formula K b Ammonia NH 3 1.8 × 10 -5 Methylamine CH 3 NH 2 4.38 × 10 -4 Ethylamine C 2 H 5 NH 2 5.6 × 10 -4 Aniline C 6 H 5 NH 2 3.8× 10 -10 Pyridine C 5 H 5 N 1.7 × 10 -9 Not ote: e: The stronger the base, the larger the K b . Cha hapt pter er 7: Acid ids s and Ba Bases ses 10
Strength of Acids/Bases Student Question 2- and HSO 3 - are 4.8×10 -13 The K a values for HPO 4 and 6.3×10 -8 respectively. Therefore, it follows 2- is a _____ acid than HSO 3 - and PO 4 that HPO 4 3- is a _____ base than SO 3 2- . a) weaker, weaker b) weaker, stronger c) stronger, weaker d) stronger, stronger Cha hapt pter er 7: Acid ids s and Ba Bases ses 11
Strength of Acids/Bases Is there a relationship between K a and K b ? General Weak Acid Equilibrium Equation HA(aq) ⇌ H + (aq) + A - (aq) 𝐼 + 𝐵 − 𝐿 𝑏 = 𝐼𝐵 General Weak Base Equilibrium Equation B(aq) + H 2 O(l) ⇌ BH + (aq) + OH - (aq) 𝐶𝐼 + 𝑃𝐼 − 𝐿 𝑐 = 𝐶 Water Equilibrium Equation H 2 O(l) ⇌ H + (aq) + OH - (aq) 𝐿 𝑋 = 𝐼 + 𝑃𝐼 − = 1.0 × 10 −14 Not ote: e: K W is known as the ion product constant. Cha hapt pter er 7: Acid ids s and Ba Bases ses 12
pH/pOH Scale How do you tell if a solution acidic, basic, or neutral? [H + ] = [OH - ] neutral [H + ] > [OH - ] acidic [H + ] < [OH - ] basic Are the [H + ] and [OH - ] related? 𝐿 𝑋 = 𝐼 + 𝑃𝐼 − = 1.0 × 10 −14 For neutral solutions 𝐼 + = 𝑃𝐼 − = 1.0 × 10 −7 [H + ] > 1.0×10 -7 and [OH - ] < 1.0×10 -7 acidic [H + ] < 1.0×10 -7 and [OH - ] > 1.0×10 -7 basic How do you calculate pH? pH=-log[H + ] pH = 7 neutral pH < 7 acidic pH > 7 basic Cha hapt pter er 7: Acid ids s and Ba Bases ses 13
pH/pOH Scale Theoretical pH of rain Milk of magnesia Most acidic rainfall recorded in U.S. Average seawater Lye Vinegar Alkaline Neutral soil Acidic soil (above 7) (5.5-6.5) Tomato juice Milk Battery acid Ammonia Human Apples blood Lemon juice Baking soda 14 Cha hapt pter er 7: Acid ids s and Ba Bases ses
pH/pOH of Strong Acids/Bases Student Question Calculate the pH of 0.25 M barium hydroxide. a) 0.60 b) 13.10 c) 13.40 d) 13.70 e) None of the above Cha hapt pter er 7: Acid ids s and Ba Bases ses 15
pH/pOH of Weak Acids/Bases Student Question What is the pH of a 0.18 M base solution whose conjugate acid has a K a = 2.8 x10 -8 ? a) 3.59 b) 9.85 c) 10.40 d) 13.25 e) None of the above Cha hapt pter er 7: Acid ids s and Ba Bases ses 16
Acid/Base Properties of Salts Student Question Is NH 4 C 2 H 3 O 2 : Helpful Information: K b NH 3 = 1.8×10 -5 and K a HC 2 H 3 O 2 = 1.8×10 -5 a) Acid b) Base c) Neutral d) More information needed Cha hapt pter er 7: Acid ids s and Ba Bases ses 17
Acid Rain pH water ~7, pH of unpolluted rain ~5.7 pH of rain in industrial areas has been recorded at ~2.4 Cha hapt pter er 7: Acid ids s and Ba Bases ses 18
Acid Rain What are the natural causes of acids in rain? Source Causes CO 2 Decomposition/Respiration/Fires NO Electrical Discharge SO 2 Volcanic Gases What are the man made causes of acids in rain? Source Causes CO 2 Fossil Fuel Combustion/Fires NO High Temperature Air Combustion SO 2 Fossil Fuel Combustion Cha hapt pter er 7: Acid ids s and Ba Bases ses 19
Acid Rain CO 2 (produced from the combustion of C or C x H y ) Coal C(s) + O 2 (g) → CO 2 (g) Gas CH 4 (g) + 2O 2 (g) → CO 2 (g) + 2H 2 O(g) CO 2 (g) + H 2 O(l) → H 2 CO 3 (aq) NO (formed from N at high temperatures) N 2 (g) + O 2 (g) heat 2NO(g) 2NO(g) + O 2 (g) → 2NO 2 (g) 3NO 2 (g) + H 2 O(l) → 2HNO 3 (aq) + NO(g) Not ote: e: The majority of the NO emissions come from automobiles. Cha hapt pter er 7: Acid ids s and Ba Bases ses 20
Acid Rain SO 2 (formed from the combustion of S) S(s) + O 2 (g )→ SO 2 (g) 2SO 2 (g) + O 2 (g) → 2SO 3 (g) SO 3 (g) + H 2 O(l) → H 2 SO 4 (aq) Not ote: e: The majority of all SO 2 emissions come from the production of electricity. Not ote: e: Why are we more worried about controlling SO 2 and NO emissions for acid rain? Cha hapt pter er 7: Acid ids s and Ba Bases ses 21
Acid Rain NO Three Way Catalytic converters (1981) 2NO(g) N 2 (g) + O 2 (g) Pt or Rh SO 2 Scrubbers (in the 1990) (limestone slurries are put into the smoke stacks) CaCO 3 (s) + H 2 SO 4 (aq) CaSO 4 (aq) + H 2 O(l) +CO 2 (g) Not ote: e: Acid rain level have dropped 65% since 1976. Cha hapt pter er 7: Acid ids s and Ba Bases ses 22
Recommend
More recommend