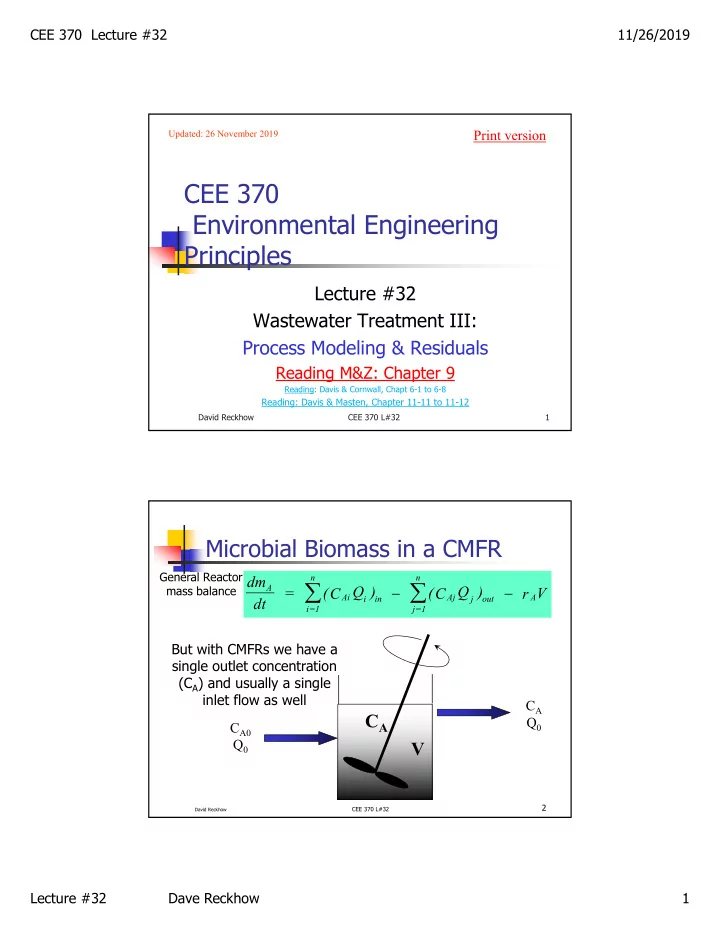

CEE 370 Lecture #32 11/26/2019 Print version Updated: 26 November 2019 CEE 370 Environmental Engineering Principles Lecture #32 Wastewater Treatment III: Process Modeling & Residuals Reading M&Z: Chapter 9 Reading: Davis & Cornwall, Chapt 6-1 to 6-8 Reading: Davis & Masten, Chapter 11-11 to 11-12 David Reckhow CEE 370 L#32 1 Microbial Biomass in a CMFR General Reactor n n dm A (C Q ) (C Q ) mass balance dt = r V Ai Aj A i in j out i=1 j=1 But with CMFRs we have a single outlet concentration (C A ) and usually a single inlet flow as well C A C A Q 0 C A0 Q 0 V 2 CEE 370 L#32 David Reckhow Lecture #32 Dave Reckhow 1
CEE 370 Lecture #32 11/26/2019 Batch Microbial Growth 0 0 General Reactor n n dM A (C Q ) (C Q ) mass balance = - r V Ai Aj A i in j out dt i=1 j=1 Batch reactors are usually filled, allowed to react, then emptied for the next batch Because there isn’t any flow in a batch reactor: 1 C A dM A = - r A V dt For 1 st order V X kX And: biomass growth dC A dt = - r A 3 CEE 370 L#32 David Reckhow Batch Microbial Growth Observed behavior Stationary Death Covered in lecture #17 Lag Exponential Growth Time 4 CEE 370 L#32 David Reckhow Lecture #32 Dave Reckhow 2
CEE 370 Lecture #32 11/26/2019 Exponential Growth model Covered in lecture #17 dX X dt gr D&M Text where, N X = concentration of microorganisms at time t t t = time = proportionality constant or specific r growth rate, [time ─ 1 ] dN/dt dX/dt = microbial growth rate, [mass per volume-time] 5 CEE 370 L#32 David Reckhow Exp. Growth (cont.) Covered in lecture #17 dX dX X dt or dt X gr gr X ln = t X o t X = X e o 6 CEE 370 L#32 David Reckhow Lecture #32 Dave Reckhow 3
CEE 370 Lecture #32 11/26/2019 Substrate-limited Growth Also known as resource-limited growth THE MONOD MODEL S dX SX and X max S max K S dt K S gr S where, max = maximum specific growth rate, [day -1 ] S = concentration of limiting substrate, [mg/L] K s = Monod or half-velocity constant, or half saturation coefficient, [mg/L] 7 CEE 370 L#32 David Reckhow Monod Kinetics Covered in lecture #17 0.5*µ m K S 8 CEE 370 L#32 David Reckhow Lecture #32 Dave Reckhow 4
CEE 370 Lecture #32 11/26/2019 Substrate Utilization & Yield Related to growth by Y, the yield coefficient Mass of cells produced H&H, Fig 11-38, pp.406 per mass of substrate utilized dX X dt Y dS S dt Just pertains to cell growth dX dS Y dt dt gr 9 CEE 370 L#32 David Reckhow Microbial Growth dX dS Y dt dt gr Monod kinetics in a chemostat (batch reactor) Substitute for dS dX SX dS XS max X max S dt K S dt Y K S gr S & Divide by Y XS Where r k e su K S dS/dt = r su = actual substrate utilization rate S e k = maximum substrate utilization rate = μ max /Y S = concentration of substrate (S e in H&H) K S = half-saturation constant Y = cell yield = dX/dS 10 CEE 370 L#32 David Reckhow Lecture #32 Dave Reckhow 5
CEE 370 Lecture #32 11/26/2019 Death Bacterial cells also die at a characteristic first order rate with a rate constant, k dX k X d dt d This occurs at all times, and is independent of the substrate concentration 11 CEE 370 L#32 David Reckhow Overall model: chemostat Combining growth and death, we have: dX dX dX dt dt dt net gr d SX k X See: M&Z equ 9.3 max d K S S And in terms of substrate utilization dX dS Y dt dt gr dX dS Y k X d dt dt net 12 CEE 370 L#32 David Reckhow Lecture #32 Dave Reckhow 6
CEE 370 Lecture #32 11/26/2019 Activated Sludge Flow Schematic Conventional X o Q+ Q r Q X X e Effluent S o Aeration S S Basin Settling Influent Tank V,X Q r X r Return activated sludge Q w X r S 13 Waste activated sludge CEE 370 L#32 David Reckhow Efficiency & HRT Efficiency of BOD removal S S 100 % E o S o Hydraulic Retention Time, HRT (Aeration Time) Same as retention time in DWT (t R ) V Actual HRT is a bit different Q Isn’t used as much in design V act Q Q R 14 CEE 370 L#32 David Reckhow Lecture #32 Dave Reckhow 7
CEE 370 Lecture #32 11/26/2019 SRT – solids retention time & R SRT: Primary operation and design parameter How long does biomass stay in system XV XV See: M&Z equ 9.10 c Q Q X Q X Q X w e w r w r Typically equals 5-15 days Q Recycle Ratio R r Q Values of 0.25-1.0 are typical 15 CEE 370 L#32 David Reckhow F:M Ratio and volumetric loading Food-to-Microorganism Ratio (F/M) F Q BOD M V X F QS o M&Z equ 9.16 M XV Typical values are 0.2-0.6 in complete mixed AS BOD volumetric Loading QS Loading o V Typically 50-120 lb BOD/day/1000ft 3 tank volume 16 CEE 370 L#32 David Reckhow Lecture #32 Dave Reckhow 8
CEE 370 Lecture #32 11/26/2019 Act. Sludge: Biomass Model dX dX dX Steady State mass balance on biomass dt dt dt net gr d dX dX SX 0 V QX Q X Q X V k X max d o e e w r K S dt dt S batch From chemostat model Incorporating the chemostat model gets: dX SX V 0 QX Q X Q X V k X o e e w r max d dt K S S And simplifying SX QX Q X Q X V k X o e e w r max d K S S Finally, we recognize that the amount of solids entering with the WW (i.e., X o ) and leaving in the treated effluent (i.e., X e ) is quite small and can be neglected 17 CEE 370 L#32 David Reckhow Biomass Model II So it becomes SX Q X V k X w r max d K S S And rearranging Q X S 1 w r k max d VX K S c S Earlier equation for SRT XV XV c Q Q X Q X Q X w e w r w r 18 CEE 370 L#32 David Reckhow Lecture #32 Dave Reckhow 9
CEE 370 Lecture #32 11/26/2019 Act. Sludge: Substrate Model Steady state mass balance on substrate dS XS max S dt Y K S dS dS 0 V QS Q S Q S V o e w From chemostat model dt dt batch Substituting and noting that Q e =Q-Q w XS max QS QS Q S Q S V o w w Y K S S And further simplifying XS Q S S V max o Y K S S 19 CEE 370 L#32 David Reckhow Merging the biomass & substrate models If we divide the previous equation by V and X Q S S S XS Q S S V max o max o Y K S VX Y K S S S Multiply both sides by Y YQ S S S o M&Z equ 9.8 max VX K S S Now insert the LH term into the 1 Q X S w r k earlier equation based on biomass max d VX K S c S 1 Q X YQ S S w r o k M&Z equ 9.9 d VX VX c 20 CEE 370 L#32 David Reckhow Lecture #32 Dave Reckhow 10
CEE 370 Lecture #32 11/26/2019 Combined model II Now recognize that Q/V is the reciprocal of the HRT 1 1 Y S S o k d X c 21 CEE 370 L#32 David Reckhow Question All else being equal, as SRT goes up: Settleability goes down 1. F/M goes down 2. Waste sludge return ratio must go down 3. Endogenous respiration becomes less 4. important Sludge yield increases 5. 22 CEE 370 L#32 David Reckhow Lecture #32 Dave Reckhow 11
Recommend
More recommend