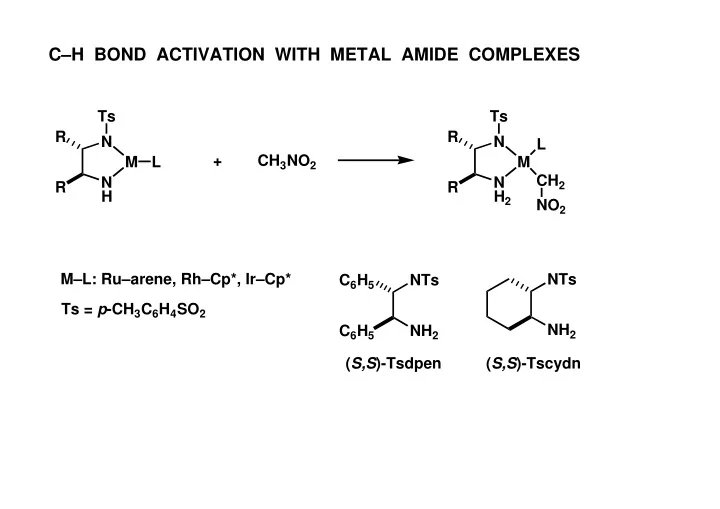

C–H BOND ACTIVATION WITH METAL AMIDE COMPLEXES Ts Ts R R N N L + CH 3 NO 2 M L M CH 2 N N R R H H 2 NO 2 M–L: Ru–arene, Rh–Cp*, Ir–Cp* NTs C 6 H 5 NTs Ts = p -CH 3 C 6 H 4 SO 2 NH 2 C 6 H 5 NH 2 ( S,S )-Tsdpen ( S,S )-Tscydn
NITROALDOL REACTION WITH CHIRAL METAL COMPLEXES Ts R Ts N C 6 H 5 M N CH 2 N M R δ− H NO 2 N H H NO 2 C 6 H 5 O H R H δ+ R δ+ H O δ− electrophilic nucleophilic activation attack CH 3 NO 2 NO 2 Ts HO H R N R M N R H M: Ru, Rh, Ir
NITROALDOL REACTION WITH CHIRAL METAL COMPLEXES O OH NO 2 chiral cat H S + CH 3 NO 2 2-methyl-2-butanol 30 ˚C, 24 h yield, % ee, % chiral cat 37 40 Ru[( S,S )-Tsdpen]( p -cymene) Cp*Rh(CH 2 NO 2 )[( S,S )-Tscydn] 29 0 25 0 Cp*Ir(CH 2 NO 2 )[( S,S )-Tscydn] Conditions: cat:aldehyde:CH 3 NO 2 = 1:50,100, [aldehyde] = 0.2 M
SOLVENT EFFECT FOR ASYMMETRIC NITROALDOL REACTION O OH chiral Ru cat NO 2 + CH 3 NO 2 C 6 H 5 H C 6 H 5 S solvent 24 h, 30 ˚C chiral Ru cat: Ru[( S,S )-Tsdpen]( p -cymene) solvent conv, % yield, % ee, % 2-methyl-2-butanol 88 37 40 2-propanol 80 30 30 methanol 100 13 17 toluene 34 10 30 6 4 39 THF Conditions: Ru:aldehyde:CH 3 NO 2 = 1:50:100, [aldehyde] = 0.2 M
EFFECTS OF THE AMOUNT OF CH 3 NO 2 OH CH 3 NO 2 , equiv temp, ˚C conv, % yield, % ee, % NO 2 C 6 H 5 S 2 30 88 37 40 2 45 32 33 50 10 63 56 36 5 chiral Ru cat: Ru[( S,S )-Tsdpen]( p -cymene) 5 30 81 65 26 10 100 83 7 30 Conditions: Ru:aldehyde = 1:50, [aldehyde] = 0.2 M in 2-methyl-2-butanol
TIME DEPENDENCE OF EE VALUES 100 100 conv, % 80 80 ee, % conv, % ee, % 60 60 40 40 20 20 0 0 0 10 30 20 time, h chiral Ru cat: Ru[( S,S )-Tsdpen]( p -cymene) Condition: Ru:aldehyde:CH 3 NO 2 =1:50:100, [aldehyde] = 0.2 M in 2-methyl-2-butanol, 30 ˚C
ASYMMETRIC NITROALDOL REACTION OF ALDEHYDES O OH chiral Ru cat + CH 3 NO 2 NO 2 R H R 2-methyl-2-butanol S 24 h S/C = 50 chiral Ru cat: Ru[( S,S )-Tsdpen]( p -cymene) R ee, % temp, °C CH 3 NO 2 , equiv yield, % C 6 H 5 (CH 2 ) 2 10 5 56 36 30 2 20 20 C 6 H 5 C 6 H 11 18 75 30 2 Conditions: [aldehyde] = 0.2 M
REACTION OF PHENYLPROPANAL WITH NITROETHANE O chiral Ru cat + CH 3 CH 2 NO 2 C 6 H 5 H 30 ˚C, 24 h 2-methyl-2-butanol 48% yield chiral Ru cat: Ru[( S,S )-Tsdpen]( p -cymene) OH OH R S + C 6 H 5 C 6 H 5 S S NO 2 NO 2 syn anti 1:1 51% ee 33% ee Ru:aldehyde:nitroethane = 1:50:100, [aldehyde] = 0.2 M
CONCLUSION O OH chiral Ru cat + R'CH 2 NO 2 NO 2 * R H R * 2-methyl-2-butanol 10–30 °C, 24 h R' S/C = 50 18–56% yield 36–75% ee R: C 6 H 5 (CH 2 ) 2 , C 6 H 5 , C 6 H 11 R'CH 2 NO 2 : CH 3 NO 2 , CH 3 CH 2 NO 2 chiral Ru cat: Ru[( S , S )-Tsdpen]( p -cymene) ACKNOWLEDGEMENTS Prof. M. Shibasaki (Tokyo Univ. )
Recommend
More recommend