
DOI: 10.1021/jacs.5b04210 By Biswajit Mondal 27-06-2015
Background: From Aggregation-Induced Emission of Au(I)− Thiolate Complexes to Ultrabright Au(0 )@Au(I)− Thiolate Core−Shell Nanoclusters Luo et al. J. Am. Chem. Soc. 2012 , 134 , �666�− 16670.
Introduction: Semiconductor quantum dots and the organic dye molecule have received attention in technological application as diode display and luminescent sensor. Xie and his co-worker have synthesized Au 22 (SG) 18 and shown that luminescence quantum yield of 8% and the origin of luminescence in them still remain unclear. Luminescence of Au(l)-thiolate complex depends on the solvent-induced and the cation-induced aggregation and this enhanced luminescence is called aggregation-induced emission(AIE). In this report they present a novel strategy to dramatically enhance the luminescence efficient of gold cluster based on the core-shell structure of Au 22 (SG) 18 . They have probed the origin of the luminescence in Au 22 (SG) 18 . Luminescence arises from the ligand to metal-metal charge transfer state of gold shell.
In this paper: In this paper they have synthesized Au 22 (SG) 18 cluster and they have done the temperature dependent and time resolved PL measurement of Au 22 (SG) 18 cluster and the TOA-Au 22 .
Result and discussion: Figure 1. (a) ESI mass spectrum of Au 22 (SG) 18 clusters obtained from the synthesis. The inset shows a comparison between the experimental data (gray line) and the calculated isotope pattern (black line) of [Au 22 (SG) 18 -5H] 5 − . (b) Absorption spectrum and (c) excitation and emission spectra of Au 22 (SG) 18 in water.
Figure 2. (a) Temperature-dependent photoluminescence spectra of Au 22 (SG) 18 clusters at different temperatures. Inset shows the plot of PL maximum (black circles) and normalized PL intensity (red circles) as a function of temperature. (b) PL decay traces of Au 22 (SG) 18 clusters as a function of temperature. Inset shows the average lifetime as a function of temperature.
Figure 3. Schematic diagram showing the excited state relaxation dynamics in Au 22 (SG) 18 clusters in water. T 1 and T 2 represent LMMCT states of the gold shell. T 2 states are destabilized by freezing or rigidifying the gold shell.
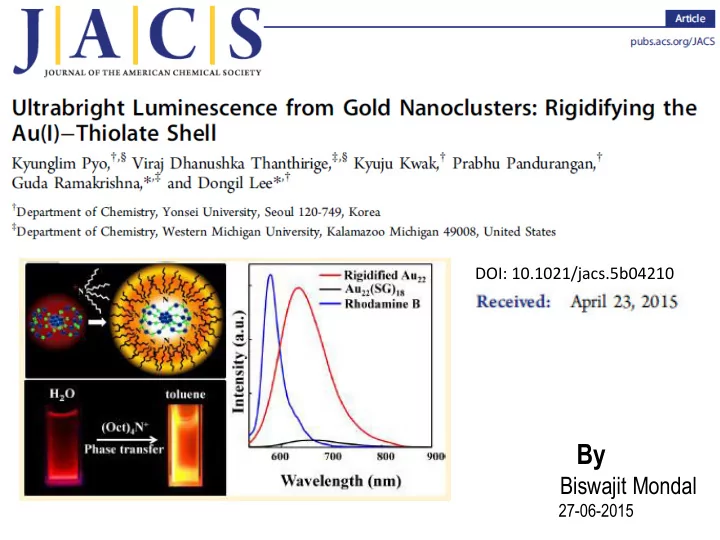
Figure 4. (a) Schematic of binding TOA to Au 22 (SG) 18 clusters (Au, blue; S, green), (b) digital photograph of Au 22 (SG) 18 in water and TOA-Au 22 clusters in toluene under long-wavelength UV lamp irradiation (365 nm), and (c) luminescence spectra of Au 22 (SG) 18 in water and TOA-Au 22 in toluene. Also, shown in the graph is the fluorescence spectrum of Rhodamine B (RhB, QY = 31%) with the same optical density.
Figure 5. (a) Luminescence spectra of Au 22 (SG) 18 paired with quaternary ammonium cations with different chain length: Au 22 (SG) 18 in water (black) and CTA- (pink), TMA- (blue), TOA- (red), and TDA-paired (green) Au 22 clusters in toluene. All cluster solutions have the same absorbance (0.025) at 514 nm and are excited at 514 nm. (b) Luminescence of TOA-Au 22 clusters in various solvents with different dielectric constants: toluene ( ε = 2.38), dichloromethane ( ε = 8.93), ethanol ( ε = 24.5), methanol ( ε = 32.7), and acetonitrile ( ε = 37.5) and Au 22 (SG) 18 in water ( ε = 80).
Conclusion: They have shown that it is possible to achieve luminescence quantum yield greater than 60% by rigidifying the gold shell by lowering the medium temperature or binding with bulky group. Aurophilic effect in the gold shell and the LMMCT relaxation is important for the higher luminescence.
Future direction: Using this concept we can increase the luminescence of the gold cluster. And this highly luminescence cluster can be used as biomedical imaging, display application. This cluster also can be used for the detection of protein.
Thank you
Recommend
More recommend