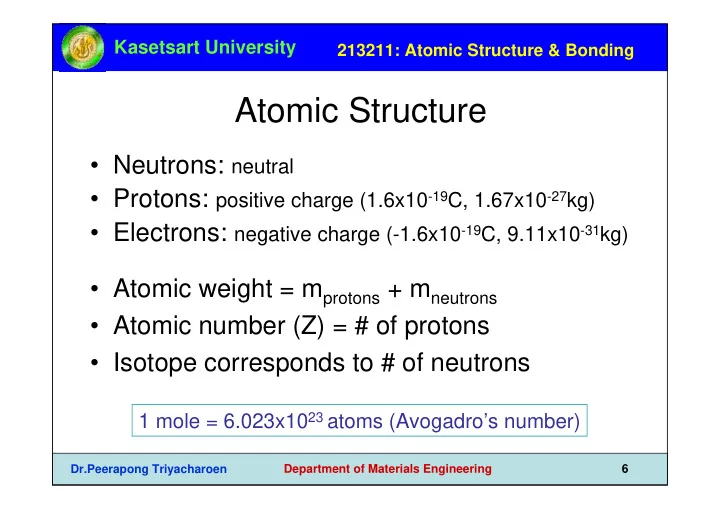

Kasetsart University 213211: Atomic Structure & Bonding Atomic Structure • Neutrons: neutral • Protons: positive charge (1.6x10 -19 C, 1.67x10 -27 kg) • Electrons: negative charge (-1.6x10 -19 C, 9.11x10 -31 kg) • Atomic weight = m protons + m neutrons • Atomic number (Z) = # of protons • Isotope corresponds to # of neutrons 1 mole = 6.023x10 23 atoms (Avogadro’s number) Dr.Peerapong Triyacharoen Department of Materials Engineering 6
Kasetsart University 213211: Atomic Structure & Bonding Quantum Mechanic: Bohr Atom orbital electrons: n = principal quantum number 2 1 n=3 Nucleus: Z = # protons = 1 for hydrogen to 94 for plutonium N = # neutrons Dr.Peerapong Triyacharoen Department of Materials Engineering 7
Kasetsart University 213211: Atomic Structure & Bonding Quantum Mechanic: Wave Mechanics • Quantum Numbers – Principle quantum number n = K, L, M, N, O, … – Subsidiary quantum number or Subshell l = s, p, d, f – Magnetic quantum number or Energy States m l = 1, 3, 5, 7 – Electron spin quantum number or Spin Moment m s = +½, -½ Structure of an atom = Electron Configuration Electrons occupy the outermost filled shell = Valence Electrons Dr.Peerapong Triyacharoen Department of Materials Engineering 8
Kasetsart University 213211: Atomic Structure & Bonding Electron Energy States • Electrons – have discrete energy states – tend to occupy lowest available energy state Increasing energy 4p n=4 3d 4s 3p n=3 3s 2p n=2 2s n=1 1s Dr.Peerapong Triyacharoen Department of Materials Engineering 9
Kasetsart University 213211: Atomic Structure & Bonding Expected Electron Configurations Element Atomic # Electron configuration Hydrogen 1 1s 1 Helium 2 1s 2 (stable) Lithium 3 1s 2 2s 1 Beryllium 4 1s 2 2s 2 Boron 5 1s 2 2s 2 2p 1 1s 2 2s 2 2p 2 Carbon 6 ... ... Neon 10 1s 2 2s 2 2p 6 (stable) Sodium 11 1s 2 2s 2 2p 6 3s 1 1s 2 2s 2 2p 6 3s 2 Magnesium 12 1s 2 2s 2 2p 6 3s 2 3p 1 Aluminum 13 ... ... 1s 2 2s 2 2p 6 3s 2 3p 6 (stable) Argon 18 ... ... ... Krypton 36 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4 6 (stable) Dr.Peerapong Triyacharoen Department of Materials Engineering 10
Kasetsart University 213211: Atomic Structure & Bonding The Periodic Table inert gases give up 1e give up 2e accept 2e accept 1e give up 3e Metal Nonmetal H He Intermediate Li Be Ne O F Mg Na Ar S Cl K Ca Sc Kr Se Br Sr Y Rb Xe Te I Cs Ba Po At Rn Fr Ra Electropositive elements : Electronegative elements : Readily give up electrons Readily acquire electrons to become + ions. to become - ions. Dr.Peerapong Triyacharoen Department of Materials Engineering 11
Kasetsart University 213211: Atomic Structure & Bonding Bonding Forces and Energies ∫ = E F dr Dr.Peerapong Triyacharoen Department of Materials Engineering 12
Kasetsart University 213211: Atomic Structure & Bonding Type of Bonding • Primary or Chemical Bonds – Ionic Bonding – Covalent Bonding – Metallic Bonding • Secondary or Physical (van der Waals) Bonds – Fluctuating induced dipole bonds – Polar molecule induced dipole bonds – Permanent dipole bonds Dr.Peerapong Triyacharoen Department of Materials Engineering 13
Kasetsart University 213211: Atomic Structure & Bonding Chemical Bonds: Ionic Bonding • Occurs between + and – ions • Requires electron transfer • Large different electronegativity required • Example: NaCl Na (metal) Cl (nonmetal) unstable unstable electron - Na (cation) + Cl (anion) stable stable Coulombic Attraction Dr.Peerapong Triyacharoen Department of Materials Engineering 14
Kasetsart University 213211: Atomic Structure & Bonding Chemical Bonds: Ionic Bonding (con.) • Predominant bonding in Ceramics NaCl MgO H CaF2 He 2.1 - Be O Li CsCl F Ne 1.5 3.5 1.0 4.0 - Mg Cl Na Ar 1.2 3.0 0.9 - Ti Cr Fe Ni Zn As K Ca Br Kr 1.5 1.6 1.8 1.8 1.8 2.0 0.8 1.0 2.8 - Sr I Xe Rb 1.0 2.5 - 0.8 Cs Ba At Rn - 0.7 0.9 2.2 Fr Ra 0.7 0.9 Give up electrons Acquire electrons Dr.Peerapong Triyacharoen Department of Materials Engineering 15
Kasetsart University 213211: Atomic Structure & Bonding Chemical Bonds: Covalent Bonding • Requires share electrons • Electronegativities are comparable • Directional bond • Example: CH 4 shared electrons – C has 4 valence e ˉ , H from carbon atom CH4 needs 4 more – H has 1 valence e ˉ , H H C needs 1 more shared electrons from hydrogen H atoms Dr.Peerapong Triyacharoen Department of Materials Engineering 16
Kasetsart University 213211: Atomic Structure & Bonding Chemical Bonds: Covalent Bonding (con.) • Molecule with nonmetals • Molecule with metals and nonmetals • Compound solids (about column IVA) H2O column IVA H2 F2 C(diamond) He H Cl2 SiC - 2.1 Li O F Ne Be C 1.0 2.0 4.0 - 1.5 2.5 Na Mg Si Cl Ar 0.9 3.0 1.2 1.8 - K Ca Ti Fe Ga Ge As Br Kr Cr Ni Zn 0.8 1.0 1.5 1.8 1.6 1.8 2.0 2.8 - 1.6 1.8 1.8 Rb I Sr Sn Xe 0.8 2.5 1.0 1.8 - Pb Cs Ba At Rn 1.8 0.7 0.9 2.2 - Fr Ra GaAs 0.7 0.9 Dr.Peerapong Triyacharoen Department of Materials Engineering 17
Kasetsart University 213211: Atomic Structure & Bonding Chemical Bonds: Metallic Bonding • Arises from a sea of donated valence electrons (1, 2, or 3 from each atom) • Nondirectional bond • Primary bond for metals and their alloys + + + Ion cores + + + Sea of valence electrons or electron cloud + + + Dr.Peerapong Triyacharoen Department of Materials Engineering 18
Kasetsart University 213211: Atomic Structure & Bonding Physical or van der Waals Bonds • Arises from atomic or molecular dipoles . • Weak in comparison to the primary bonds. Dr.Peerapong Triyacharoen Department of Materials Engineering 19
Kasetsart University 213211: Atomic Structure & Bonding Physical Bonds: Fluctuating dipoles An induced atomic dipole An electrically symmetric atom ex: liquid H2 asymmetric electron H2 H2 clouds - - + + H H H H secondary secondary bonding bonding Inert gases and electrically neutral and symmetric molecules, ex. H 2 , Cl 2 Weakest bonds Dr.Peerapong Triyacharoen Department of Materials Engineering 20
Kasetsart University 213211: Atomic Structure & Bonding Physical Bonds: Polar Molecule • Can induce dipoles in adjacent nonpolar molecules. • Bond forms between the two molecules, which the magnitude is greater than for fluctuating induced dipoles. Dr.Peerapong Triyacharoen Department of Materials Engineering 21
Kasetsart University 213211: Atomic Structure & Bonding Physical Bonds: Permanent dipoles • Exist between adjacent polar molecules. • Energy are greater than induced dipoles. • Ex: Hydrogen bonding: H – F, H – O, or H – N bond secondary bonding Hydrogen Fluoride Polymer Dr.Peerapong Triyacharoen Department of Materials Engineering 22
Kasetsart University 213211: Atomic Structure & Bonding Summary Type Bond Energy Comment Ionic Large Nondirectional (ceramics) Variable Directional (semiconductor, Covalent large – Diamond ceramics, polymer chains) small – Bismuth Variable Metallic large – Tungsten Nondirectional (metals) small – Mercury Directional (inter-molecular, inter- Secondary Smallest chain polymer) Dr.Peerapong Triyacharoen Department of Materials Engineering 23
Recommend
More recommend