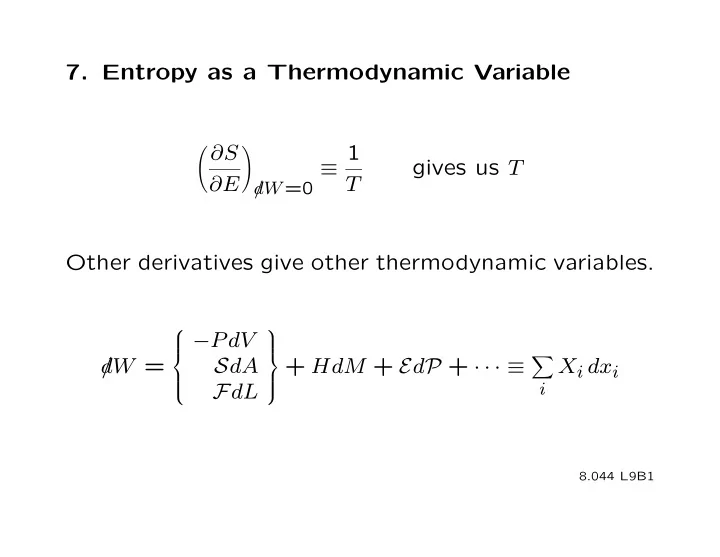

7. Entropy as a Thermodynamic Variable ∂S 1 � � gives us T ≡ ∂E d T /W =0 Other derivatives give other thermodynamic variables. ⎧ ⎫ − P dV ⎪ ⎪ ⎪ ⎪ ⎨ ⎬ d /W = S dA + HdM + E d P + · · · ≡ X i dx i ⎪ ⎪ i F dL ⎪ ⎪ ⎩ ⎭ 8.044 L9B1
We chose to use the extensive external variables (a complete set) as the constraints on Ω. Thus S ≡ k ln Ω = S ( E, V, M, · · · ) Now solve for E . S ( E, V, M, · · · ) ↔ E ( S, V, M, · · · ) We know from the 1 ST law dE | d = d /Q /W =0 utilizing the 2 ND law dE | d /W =0 ≤ T dS 8.044 L9B2
Now include the work. dE = d /Q + d /W dE ≤ T dS + d /W − P dV dE ≤ T dS + S dA + HdM + E d P + · · · F dL The last line expresses the combined 1 ST and 2 ND laws of thermodynamics. 8.044 L9B3
Solve for dS . 1 P H E dS = dE + dV − dM − d P + · · · T T T T Examine the partial derivatives of S . ∂S H ∂S = 1 = − ∂M T ∂E T E,V, P V,M, P ⎛ ⎞ ⎝ ∂S X j ∂S = P = − ⎠ ∂x j E,x i T ∂V T E,M, P = x j 8.044 L9B4
INTERPRETATION S(E,V) � ∂S � ∂S � � dS = dE + dV V ∂E ∂V V E = 1 T dE + P T dV E 8.044 L9B5
UTILITY Internal Energy ∂S ( E, V, N ) 1 = → T ( E, V, N ) ↔ E ( T, V, N ) ∂E T V Equation of State ∂S ( E, V, N ) P = → P ( E, T, V, N ) → P ( T, V, N ) ∂V T E 8.044 L9B6
Example Ideal Gas ⎧ ⎫ 3 / 2 4 E ⎪ ⎪ ⎨ ⎬ S ( E, N, V ) = k ln Φ = kN ln V πem 3 N ⎪ ⎪ ⎩ ⎭ ∂S kN {} kN P = = = ∂V {} V V T E,N PV = NkT 8.044 L9B7
COMBINATORIAL FACTS # different orderings (permutations) of K distin- guishable objects = K ! # of ways of choosing L from a set of K : K ! if order matters ( K − L )! K ! if order does not matter L !( K − L )! 8.044 L9B8a
EXAMPLE Dinner Table, 5 Chairs (places) Seating, 5 people 5 · 4 · 3 · 2 · 1 = 5! = 120 5 · 4 · 3 = 5! = 60 Seating, 3 people 2! 5 · 4 · 3 / 6 = 5! 1 Place settings, 3 people 3! = 10 2! 8.044 L9B8b
������������������������� ����������������������������������� ������ � ��� > ����� � ���� � ε ��� � ε � �� � ��� > � 8.044 L9B9
����������������� ����������������� ������������������� ������ ε � ε � ε � ������� � �� ��������������������������������� 8.044 L9B10
1 when N 1 = 0 or N N ! Ω( E ) = N 1 !( N − N 1 )! Maximum when N 1 = N/ 2 T= (or - ) S(E) S ( E ) = k ln Ω( E ) E = ε N E = ε N/2 T=0 E T>0 T<0 8.044 L9B11
ln N ! ≈ N ln N − N S ( E ) = k [ N ln N − N 1 ln N 1 − ( N − N 1 ) ln( N − N 1 ) − N + N 1 + N − N 1 ] 1 ∂S ∂S ∂N 1 k = = = [ − 1 − ln N 1 + 1 + ln( N − N 1 )] T ∂E ∂N 1 , ∂E E N af � 1 /E ⎛ ⎞ ⎛ ⎞ k N − N 1 k N = ln = ln − 1 ⎝ ⎠ ⎝ ⎠ E N 1 E N 1 8.044 L9B12
N N E/kT − 1 = e → N 1 = E/kT + 1 N 1 e EN E = EN 1 = E/kT + 1 e N 1 /N or E/ ε N 1.0 −ε /kT ~ ~ e 0.5 4 2 1 3 kT/ ε 8.044 L9B13
E/kT � 2 ∂E E e � C ≡ = Nk ( e E/kT + 1) 2 ∂T kT � 2 � 2 E Nk E � � − E/kT → Nk e low T , high T → 4 kT kT 0.5 C/Nk 0.4 0.3 0.2 0.1 1 2 3 kT/ ε 4 8.044 L9B14
p ( n ) = Ω ' p ( n ) =? n = 0 , 1 Ω In Ω ' N → N − 1 and N 1 → N 1 − n ( N − 1)! ( N 1 − n )!( N − 1 − N 1 + n )! p ( n ) = N ! N 1 !( N − N 1 )! 8.044 L9B15
( N − 1)! N 1 ! ( N − N 1 )! p ( n ) = N ! ( N 1 − n )! ( N − N 1 − 1 + n )! � �� � � �� � � �� � 1 /N 1 n = 0 N − N 1 n = 0 N 1 n = 1 1 n = 1 ⎫ p (0) = N − N 1 = 1 − N 1 ⎪ ⎪ N N ⎪ ⎬ p (0) + p (1) = 1 ⎪ p (1) = N 1 = [ e E/kT + 1] − 1 ⎪ ⎪ ⎭ N 8.044 L9B16
1 p(n) p(0) 0.5 p(1) 0 1 n 1 2 3 kT/ ε 4 EN E = (0) N p (0) + ( E ) N p (1) = E/kT + 1 e But we knew E , so we could have worked back- wards to find p (1). 8.044 L9B17
����������������������� ���������������� ������ ٠������������ ��������������������� ����������������������� ��������� ٠� /٠� ������������������� � � � � ��� � � � � � � ��� ���� 8.044 L9B18
The microcanonical ensemble is the starting point for Statistical Mechanics. • We will no longer use it to solve problems. • We will develop our understanding of the 2 ND law. • We will derive the canonical ensemble, the real workhorse of S.M. 8.044 L9B19
MIT OpenCourseWare http://ocw.mit.edu 8.044 Statistical Physics I Spring 2013 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms.
Recommend
More recommend