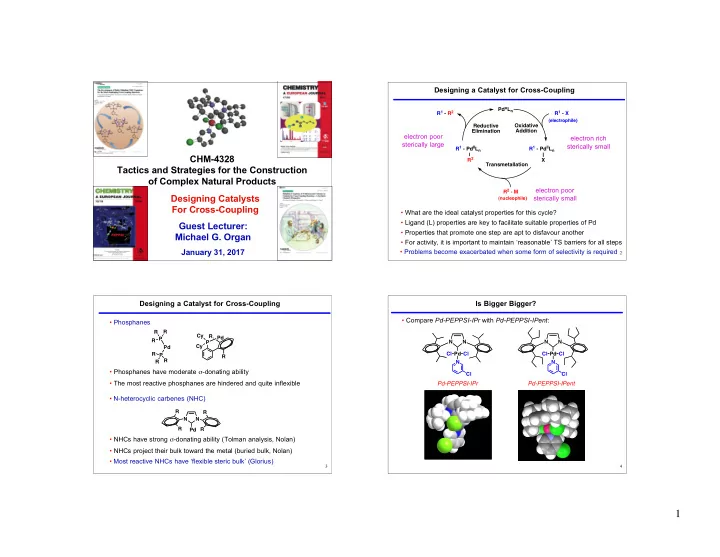

Designing a Catalyst for Cross-Coupling Pd o L n R 1 - R 2 R 1 - X (electrophile) Reductive Oxidative Elimination Addition electron poor electron rich sterically large sterically small R 1 - Pd ll L n R 1 - Pd ll L n CHM-4328 R 2 X Transmetallation Tactics and Strategies for the Construction of Complex Natural Products electron poor R 2 - M Designing Catalysts sterically small (nucleophile) For Cross-Coupling • What are the ideal catalyst properties for this cycle? • Ligand (L) properties are key to facilitate suitable properties of Pd Guest Lecturer: • Properties that promote one step are apt to disfavour another Michael G. Organ • For activity, it is important to maintain ‘reasonable’ TS barriers for all steps January 31, 2017 • Problems become exacerbated when some form of selectivity is required 1 2 Designing a Catalyst for Cross-Coupling Is Bigger Bigger? • Compare Pd-PEPPSI-IPr with Pd-PEPPSI-IPent : • Phosphanes R R Cy R Pd P R P N N N N Cy Pd R Cl Pd Cl Cl Pd Cl P R R R N N • Phosphanes have moderate σ -donating ability Cl Cl • The most reactive phosphanes are hindered and quite inflexible Pd-PEPPSI-IPr Pd-PEPPSI-IPent • N-heterocyclic carbenes (NHC) R R N N R R Pd • NHCs have strong σ -donating ability (Tolman analysis, Nolan) • NHCs project their bulk toward the metal (buried bulk, Nolan) • Most reactive NHCs have ‘flexible steric bulk’ (Glorius) 3 4 1 1
Negishi Coupling Substrate Study With Pd-PEPPSI-IPent Suzuki-Miyaura Coupling Substrate Study With Pd-PEPPSI-IPent Pd-PEPPSI (2 mol %) Pd-PEPPSI (2 mol %) Ar-Br BrMgAr X n ZnAr Ar - Ar KO t Bu, t BuOH, 65 o C, 24 h R 1 - B(OH) 2 R - R 1 ZnX 2 (equiv) NMP, 2.5h, R - Cl + (equiv) Temp, Time THF, RT, 20 min. (2 equiv) O O O ArMgBr (1.6 equiv) O ArMgBr (1.2 equiv) ZnBr 2 (1.6 equiv) Si ZnCl 2 (1.4 equiv) 50 o C, 8h IPent: 70 % IPent: 61 % IPent: 65 % IPent: 49 % IPent: 80 % 40 o C, 24h ArMgBr (1.6 equiv) ArMgBr (1.2 equiv) IPr : 34 % IPr: <2 % IPr: 0 % IPr: 0 % IPr: 34 % IPr: 0 % ZnBr 2 (1.6 equiv) IPent : 73 %, ZnCl 2 (1.4 equiv) ArMgBr (1.2 equiv) IPent : 94 %, 50 o C, 24h 0 o C, 8h RT, 16h ZnCl 2 (1.4 equiv) 0 o C, 8h O IPr : 13 %, IPent : 80 % IPent : 99 % IPent : 90 % RT, 4h F IPent : 80 % IPent : 85 % O HO O N OH H 2 N IPent: 78 % IPent: 95 % IPent: 95 % IPent: 88 % IPent: 89 % ArMgBr (2.6 equiv) IPr: 32 % IPr: 47 % IPr: 0 % IPr: 0 % ArMgBr (1.2 equiv) ZnBr 2 (3.0 equiv) ArMgBr (1.6 equiv) 50 o C, 24h ZnCl 2 (1.6 equiv), 50 o C, 16h, ZnCl 2 (1.4 equiv), NaH (1.0 equiv) • Pd-PEPPSI-IPent is more reactive than Pd-PEPPSI-IPr 50 o C, 24h, IPr : 43 %, IPent : 80 % IPr : 1 %, IPent : 57 % IPr : 43 %, IPent : 80 % 5 6 Organ, M. G.; Çalimsiz, S; Sayah, M.; Hoi, K. H. Angew. Chem. Int. Ed. 2009 , 48 , 2383-2387. • Hindered biaryls accessible at room temperature - and even lower! Cross-Coupling With Secondary Alkylzincs Cross-Coupling With Secondary Alkylzincs If R 2 << R 1 (H) this is the lower energy • Top 100 Drugs by 2011 US Retail Sales: Pd-alkyl and thermodynamic sink R-X R O OH OH SO 2 Me H Pd o L n Pd ll L n oxidative N N reductive PhHN N R 1 addition elimination OH CO 2 H N R 2 N CO 2 H R R 1 R Pd ll L n R Pd ll L n migratory R OH OH O R 1 F insertion R 2 Lipitor (Pfizer) Effexor (Pfizer) Crestor (AstraZeneca) R 2 H X R 1 transmetallation • Molecules with higher sp 3 content are gaining in interest in drug discovery R 2 • Fraction of sp 3 C: at discovery stage: 0.36 ; H L of successful drugs: 0.47 (a 31% increase!) Pd R 1 ZnX R • Greater binding specificity and improved bioavailability relative to biaryls R 1 ZnX 2 β -hydride R 2 elimination R 2 Lovering, F. et al J. Med. Chem . 2009 , 52 , 6752; Med. Chem. Commun . 2013 , 4 , 515 Problem: there are few ways to quickly and reliably install alkyl groups onto • Catalyst must be designed to favour RE over BHE aromatic systems, especially when the desired substituent is secondary . • What about catalyst size? A larger ligand should favour RE Solution: Cross-coupling of secondary alkyls? 7 8 2 2
Cross-Coupling With Secondary Alkylzincs Cross-Coupling With Secondary Alkylzincs • Test Reactions with Isopropylzinc Bromide and Aromatic Halides: • What about electronic effects? • An electron poor Pd centre should favour reductive elimination Pd-PEPPSI cat. Br (1 mol%) + + R R R BrZn O O Cl Cl THF / Toluene, branched (B) linear (L) RT, 30 min. (normal) (rearranged) N N N N N N Cl Pd Cl Cl Pd Cl Cl Pd Cl Ar-Br Cat Yield B:L Ar-Br Cat Yield B:L N N N Cl Cl Cl 4-CO 2 CH 3 IPr 99 6 : 1 3-OCH 3 IPr 31 3.5 : 1 Pd-PEPPSI-IPr Cl Pd-PEPPSI-IPr Quino Pd-PEPPSI-IPr IPent 98 40 : 1 IPent 57 34 : 1 Cl Cl 4-OCH 3 IPr 89 2.5 : 1 2-CN IPr 99 1 : 8 N N N N IPent 95 33 : 1 IPent 80 2.4 : 1 Cl Pd Cl Cl Pd Cl 3-CN IPr 77 1 : 1.4 2-OCH 3 IPr 99 1 : 9 N N IPent 84 11 : 1 IPent 46 2 : 1 Cl Cl Pd-PEPPSI-IPent Cl Pd-PEPPSI-IPent • Pd-PEPPSI-IPent resists β -hydride elimination / migratory insertion 9 10 Cross-Coupling With Secondary Alkylzincs Cross-Coupling With Secondary Alkylzincs • Are the causative effects actually electronic in origin? • What about electronic effects? • Tolman Electronic Parameter Analysis (Schrock, Nolan) Pd-PEPPSI cat. Br (1 mol%) X X X X + + R R R R R BrZn R 1. KO t Bu (1.2 equiv.) R R R R R THF / Toluene, THF, RT, 2h N N N N branched (B) linear (L) RT, 30 min. (+) 2. [(COD)IrCl)] 2 (0.5 equiv.) (normal) (rearranged) R R R R RT, 24 h Ir R R R R Cl CO 3. CO (g) , CH 2 Cl 2 , RT, 1h Ar-Br Cat Yield B:L Ar-Br Cat Yield B:L CO ν CO (CH 2 Cl 2 , cm - 1 ) ν CO (avg) Entry NHC Selectivity TEP (cm - ) [a] 3-CN IPr 82 1 : 1.4 2-CN IPr 58 1 : 6.6 1 IPr 1 : 1.4 2066.8, 1981.0 2023.9 2051.5 [b] IPr Cl 81 14.7 : 1 IPr Cl 66 4.3 : 1 2 IPr Cl 14.7 : 1 2071.4, 1985.1 2028.3 2054.0 [b] IPr Quino 78 13.4 : 1 IPr Quino 70 8.5 : 1 3 IPr Quino 13.4 : 1 2073.7, 1987.3 2030.5 2057.1 IPent 66 10.5 : 1 IPent 80 2.4 : 1 4 IPr Me 15 : 1 2064.5, 1978.2 2021.3 2049.3 IPent Cl 81 56 : 1 IPent Cl 56 27.1 : 1 5 IPent 10.5 : 1 2064.7, 1978.6 2021.7 2049.6 6 IPent Cl 56 : 1 2069.3, 1982.2 2025.8 2053.0 • The installation of electron-withdrawing substituents on the imidazolium [a] TEP = TEP computed using the linear regression: TEP (cm-1) = 0.8475*( ν CO(avg)) + 336.2; core dramatically impacts β -hydride elimination / migratory insertion [b] Organometallics 2008 , 27 , 202-210. 11 12 3 3
Cross-Coupling With Secondary Alkylzincs Cross-Coupling With Secondary Alkylzincs • OK, if it is not electronic (entirely at least), then what is it? • OK, if it is not electronic (entirely at least), then what is it? R R N N Cl Pd Cl N Cl • Two critical transition states (TS) R R R R N N N N Pd Pd H Ph Ph • Increasing the steric bulk around Pd has the greatest impact on BHE 13 14 Reductive Elimination TS Beta-Hydride Elimination TS Cross-Coupling With Secondary Alkylzincs Cross-Coupling With Secondary Alkylzincs • Reaction scope • Limitation: 5-membered ring heterocycles Pd-PEPPSI-IPent Cl Pd-PEPPSI-IPent Cl (2 mol %) (2 mol %) Ar - X + secAlkyl - ZnX Ar - secAlkyl Ar - X + secAlkyl - ZnX Ar - secAlkyl (1.2 equiv) THF / Toluene, (1.2 equiv) THF / Toluene, Ph RT, 30 min RT, 30 min S O O S O O N N S N OCH 3 S 3 N N N 3 N Boc 3 3 1 2 2 2 2 1 1 1 X = Br, (94 %) X = Cl, (99 %) X = Cl, 5 h, (99%) X = Br, 4 h, (84 %) X = Br, (86 %) X = Br, (88 %) X = Br, (88 %) X = Br, (56 %) N : R, > 99 : 1 N : R, > 99 : 1 N : R, >99 : 1 N : R, > 99 : 1 C3 : C2, > 2.4 : 1 C3 : C2 : C1, > 0.5 : 1 : 0.2 C3 : C2 : C1, > 0.2 : 0.7 : 1 C3 : C2, > 1.2 : 1 O O O • What is different with these systems that drives migratory insertion? N H N H • Due to bond angles, steric effects have been reduced OH F N N Pd-PEPPSI-IPent Cl Boc ZnBr Boc (2.2 equiv. RZnBr) (2 mol %) X = Cl, 16h (88 %) X = Cl, 24 h, (67%) X = Cl, 4 h, (84 %) X = Br, (98 %) + 3 N : R, >99 : 1 N : R, 56 : 1 N : R, > 99 : 1 N : R, > 99 : 1 THF / Toluene, 2 Br • All single isomers - zero isomerization! RT, 30 min (1.2 equiv) 1 C3 only isomer (95 %) • Effect appears not to be steric in origin Pompeo, M.; Hadei, N.; Froese, R. D. J.; Organ, M. G. Angew. Chem. Int. Ed. 2012 , 51 , 11354 –11357. 15 16 4 4
Recommend
More recommend