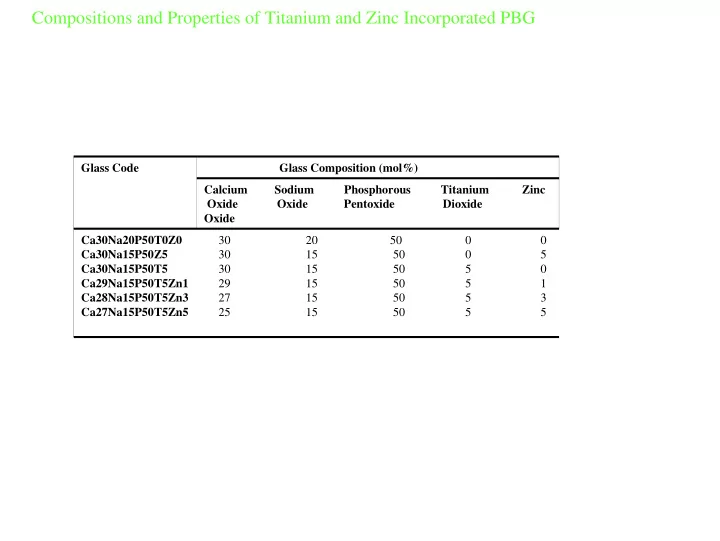

Compositions and Properties of Titanium and Zinc Incorporated PBG Glass Code Glass Composition (mol%) Calcium Sodium Phosphorous Titanium Zinc Oxide Oxide Pentoxide Dioxide Oxide 30 20 50 0 0 Ca30Na20P50T0Z0 30 15 50 0 5 Ca30Na15P50Z5 30 15 50 5 0 Ca30Na15P50T5 29 15 50 5 1 Ca29Na15P50T5Zn1 27 15 50 5 3 Ca28Na15P50T5Zn3 25 15 50 5 5 Ca27Na15P50T5Zn5
1 and 7 Days HOS Viability
1 Day HOS Attachment
7 Days HOS Attachment
Effect of Glass Extract on Cell Proliferation 1.6 Day 1 1.5 Day 4 1.4 Day 7 1.3 1.2 1.1 1 0.9 0.8 Relative Growth 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 -0.1 T0Z0 Z5 Thermanox T5 T5Z1 T5Z3 T5Z5 -0.2 -0.3 -0.4 -0.5 Glass Extract
Effect of Composition on Cell Proliferation Day 1 1.6 Day 3 Day 5 1.5 Day 7 1.4 Day 10 1.3 Day 14 1.2 Day 21 1.1 1 0.9 Relative Growth 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 0 5 x 5 1 3 5 -0.1 Z Z o T Z Z Z 0 n 5 5 5 T a T T T m r e h T -0.2 Glass Code
Summary ♦ Novel gallium-doped phosphate based glasses(PBGs) were prepared using conventional melt quenching method Glass code Glass composition (mol%) Calcium Sodium Phosphorous Oxide Oxide Pentoxide Ga Ca 16 Na 39 P 45 16 39 45 0 Ca 16 Na 38 P 45 Ga 1 16 38 45 1 Ca 16 Na 36 P 45 Ga 3 16 36 45 3 Ca 16 Na 34 P 45 Ga 5 16 34 45 5 ♦ Both density and glass transition temperature ( Tg ) was found to increase as the Ga content increased in the glass system ♦ Degradation rate of glasses in water found to decrease with the increase of Ga content . pH was found to maintain at neutral for 3 and 5 mol% Ga doped PBGs but showed initial increase for 0 and 1 mol% Ga doped PBGs ♦ Calcium and sodium ion release from the glasses were analysed using IC ♦ Gallium and phosphorous release from the glasses were analysed using ICP-MS ♦ Antimicrobial activity of these glasses against E. coli, P. aeruginosa and S. aureus were tested by disc diffusion assay ♦ The antibacterial effect was found to decrease as the Ga content increased in the glass system, with Ca 16 Na 38 P 45 Ga 1 showing maximum bactericidal effect against E. coli, P. aeruginosa and S. aureus
Characterization of Ga-doped phosphate glasses Density Tg 2.8 375 370 2.75 365 Glass Transition Temperature ( 0 C) 2.7 360 Density (g.cm -3 ) 2.65 355 350 2.6 345 2.55 340 2.5 335 0 0.5 1 1.5 2 2.5 3 3.5 4 4.5 5 Gallium Oxide Content (mol%) Density and glass transition temperatures as a function of gallium oxide content (mol%)
Dissolution and pH analysis of the 0, 1, 3 and 5 mol% Ga-doped PBGs. 6 Ga0 Ga1 Ga3 Ga5 y = 0.0417x 5 R 2 = 0.9792 4 2 ) Weight loss (mg.mm y = 0.0236x 3 R 2 = 0.9866 2 y = 0.0073x 1 R 2 = 0.9851 y = 0.0037x (a) Weight loss vs. time R 2 = 0.995 0 0 24 48 72 96 120 144 Time(hrs) 9 Ga0 Ga1 Ga3 Ga5 8 pH pH vs.time 7 6 0 20 40 60 80 100 120 Time(hrs)
Cumulative ion release profiles of the 0, 1, 3 and 5 mol% Ga-doped PBGs y = 2.7145x y = 0.7058x R 2 = 0.936 R 2 = 0.9555 250 0 1 3 5 60 0 1 3 5 Na ion release (ppm) 200 y = 0.4049x 50 Ca ion release (ppm) R 2 = 0.9843 y = 1.3007x R 2 = 0.9531 150 40 30 100 y = 0.0983x 20 y = 0.2764x 50 R 2 = 0.9167 R 2 = 0.9477 y = 0.1765x 10 y = 0.063x R 2 = 0.1549 0 R 2 = 0.8143 0 0 24 48 72 96 120 144 0 24 48 72 96 120 144 (a) Time(hrs) (b) Time(hrs) y = 50.905x 60 R 2 = 0.9489 0 1 3 5 5000 0 1 3 5 y = 0.3935x 50 4500 R 2 = 0.8336 y = 35.016x Ga ion release (ppm) 4000 R 2 = 0.9624 P ion release (ppm) 40 3500 3000 30 y = 0.2334x 2500 R 2 = 0.9313 2000 20 1500 y = 0.1143x y = 6.0693x R 2 = 0.9973 1000 R 2 = 0.9217 10 500 y = 1.2474x y = 0.0024x R 2 = 0.9974 R 2 = 0.9798 0 0 (c) 0 24 48 72 96 120 144 (d) 0 24 48 72 96 120 144 Time(hrs) Time(hrs) (a) calcium ion release vs. time (b) sodium ion release vs. time (c) phosphorous ion release vs. time and (d) gallium ion release vs. time
Antimicrobial assay of Ga-doped phosphate glasses E.coli P.aeruginosa S.aureus MRSA C.difficle 30 25 20 Diameter (mm) 15 10 5 0 1 3 5 Ga (mol%) Disc diffusion assay conducted on 0, 1, 3 and 5 mol% Ga-doped PBGs against Staphylococcus aureus , Escherichia coli , Pseudomonas aeruginosa , methicillin-resistant Staphylococcus aureus (MRSA) and Clostridium difficile
Effect of Ga-doped phosphate glasses on P. aeruginosa control C16Ga0 C16Ga1 C16Ga3 8.25 8 Log CFU/mm 2 7.75 7.5 7.25 7 0 4 12 Time(hrs) The effect of 0, 1 and 3 mol% Ga-doped PBGs on the viability of suspensions of Pseudomonas aeruginosa.
Summary ♦ P. aeruginosa causes a range of biofilm-associated infections such as Cystic fibrosis ♦ Phosphate Based Glasses doped with silver were used in the CDFF to study their effect on these biofilms Glass code Glass composition (mol%) Calcium Sodium Phosphorous Oxide Oxide Pentoxide Ag Ca 30 Na 20 P 50 30 20 50 0 Ca 30 Na 17 P 50 Ag 3 30 17 50 3 Ca 30 Na 15 P 50 Ag 5 30 15 50 5 Constant Depth Film Fermentor (CDFF) ♦ Biofilms growth were analyzed using scanning electron microscope ( SEM ) ♦ The effect of silver on the biofilms were tested by counting P. aeruginosa colony-forming units ( CFUs ) at different time ♦ ‘ Live and dead’ cell distribution in the biofilm was determined by confocal laser scanning microscope ( CLSM ).
The effect of silver on the biofilms; in terms of P. aeruginosa colony-forming units HA Ag- 5 mol% Ag 8 7 -2 ) Log (CFU.mm 6 5 4 0 25 50 75 100 125 150 Time (hours) Log 10 CFU/mm 2 of P. aeruginosa in biofilms formed on hydroxyapatite discs (HA), Ca 30 Na 20 P 50 disc (Ag-), and Ca 30 Na 15 P 50 Ag 5 discs (5 mol% Ag).
From Mg-substituted hydroxyapatites to bones: first 43 Ca NMR investigations
1- Mg-HA Crystal structure of hydroxyapatite Ca(1) site Ca 10 (PO 4 ) 6 (OH) 2 O 3 HO - columns O 2 O 1 PO 4 3- O 1 O 3 O 2 P 2 1 / b O 2 O 1 c b O 3 a Ca Ca(2) site P O O 3 H O 3’ O 1 OH Ca(1) O 2 Ca(2) O 3 O 3’
2- Mg-HA Synthesis and characterisation of Mg-substituted HA Ca(NO 3 ) 2 .6H 2 O 1/ pH ~ 10.0, 100 ˚ C, 5h 2/ centrifugation / washing + “Ca 10-x Mg x (PO 4 ) 6 (OH) 2 ” + NH 4 H 2 PO 4 Mg(NO 3 ) 2 .4H 2 O 3/ 100˚C drying under vacuum, 12h x = 0, 0.25, 0.5, 0.75, 1.0, 1.25, 1.5, 2.0 Ca(1) site Ca(2) site Mg 2+ ? O 3 O 2 O 1 O 3 O 3’ O 1 O 1 O 3 OH O 2 O 2 O 2 Elemental analyses, SEM, XRD, TG, O 1 DTA, IR, Raman, SSA measurements O 3 O 3’ O 3 High-temperature XRD Catalytic studies Solid State NMR ( 31 P, 1 H, 43 Ca , 25 Mg )
3- Mg-HA High-temperature XRD characterisation of Mg-substituted HA 1100 1050 Ca(1) site Ca(2) site 1000 O 3 950 O 2 DTA T (˚C) O 1 O 3 900 XRD 850 O 3’ O 1 O 1 800 OH O 3 O 2 750 O 2 O 2 700 0 2.5 5 7.5 10 12.5 15 17.5 20 22.5 O 1 O 3 x(Mg) (%) O 3 O 3’ Temperature of formation of whitlockite depends on Mg-content Indication of the site of incorporation of Mg ? - Mg known to enter the Ca(5) site in whitlockite - Environment of one Ca site similar in HA (Ca(1)) and in whitlockite (Ca(5)) - Transformation into whitlockite favoured by higher Mg contents Mg seems to have been incorporated in Ca(1) site J. Knowles, EDI, London
4- Mg-HA Catalytic properties of Mg-substituted HA CH 3 H 3 C alkaline surface H 3 C C C CH C O + HC CH Mg-HA H 3 C OH acetylene acetone methylbutynol (MBOH) Reaction catalysed by alkaline surfaces (presence of OH groups), HA have been shown to catalyse this reaction In Mg-HA, the reactivity of the OH group is expected to change if Mg enters the Ca(2) site O 3 O 3’ O 1 OH No clear correlation between the catalytic activity and the O 2 % of Mg in the lattice O 3 O 3’ Confirmation that Mg has been incorporated in Ca(1) site H. Pernot et al , UPMC, Paris
5- Mg-HA 43 Ca solid-state NMR of Ca 10 (PO 4 ) 6 (OH) 2 Natural Quadrupole moment Relative Isotope Spin abundance(%) Q receptivity 43 Ca 4.19*10 -5 7/2 0.135 -4.08 18.75 T 4 kHz, D1 = 0.1s RAPT-1pulse NS = 1197824, LB = 80 14.1 T 4 kHz, D1 = 1s fwhm = 963 Hz RAPT-1pulse NS = 81 000, LB = 80 8.45 T 4 kHz, D1 = 1s RAPT-1pulse fwhm = 1042 Hz NS = 180 000, LB = 80 200 150 100 50 0 -50 -100 -150 -200 -250 ppm Quadrupolar interaction main cause of line-broadening
Recommend
More recommend