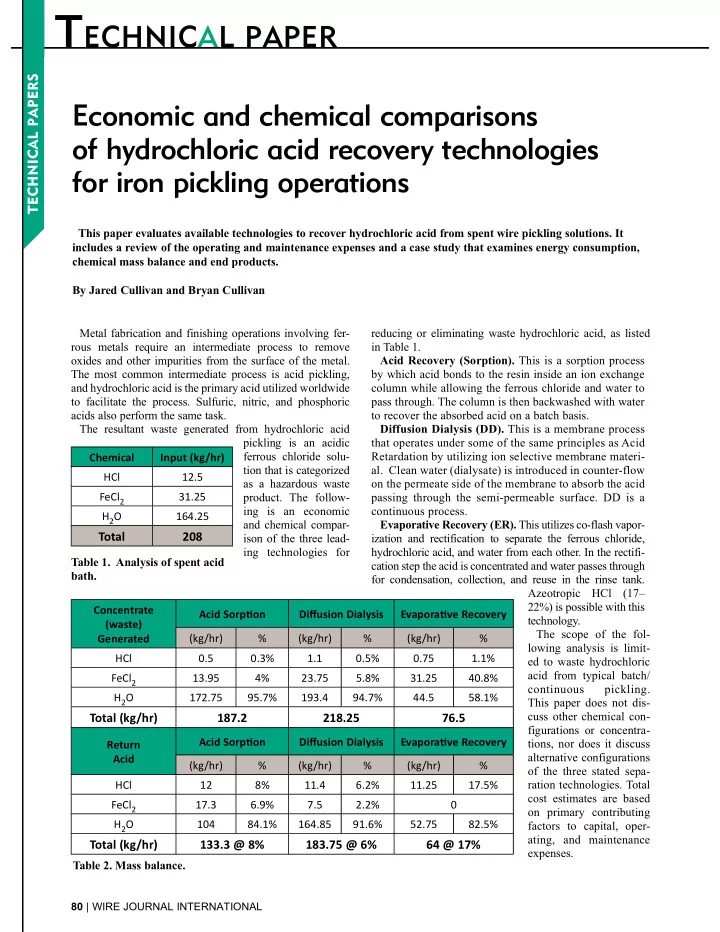

T ECHNIC T ECHNICAL PAPER ECHNICAL PAPER Economic and chemical comparisons of hydrochloric acid recovery technologies for iron pickling operations This paper evaluates available technologies to recover hydrochloric acid from spent wire pickling solutions. It includes a review of the operating and maintenance expenses and a case study that examines energy consumption, chemical mass balance and end products. By Jared Cullivan and Bryan Cullivan Metal fabrication and finishing operations involving fer- reducing or eliminating waste hydrochloric acid, as listed rous metals require an intermediate process to remove in Table 1. oxides and other impurities from the surface of the metal. Acid Recovery (Sorption). This is a sorption process The most common intermediate process is acid pickling, by which acid bonds to the resin inside an ion exchange and hydrochloric acid is the primary acid utilized worldwide column while allowing the ferrous chloride and water to to facilitate the process. Sulfuric, nitric, and phosphoric pass through. The column is then backwashed with water acids also perform the same task. to recover the absorbed acid on a batch basis. The resultant waste generated from hydrochloric acid Diffusion Dialysis (DD). This is a membrane process pickling is an acidic that operates under some of the same principles as Acid ferrous chloride solu- Retardation by utilizing ion selective membrane materi- Chemical Input (kg/hr) tion that is categorized al. Clean water (dialysate) is introduced in counter-flow HCl 12.5 as a hazardous waste on the permeate side of the membrane to absorb the acid FeCl 2 31.25 product. The follow- passing through the semi-permeable surface. DD is a ing is an economic continuous process. H 2 O 164.25 and chemical compar- Evaporative Recovery (ER). This utilizes co-flash vapor- Total 208 ison of the three lead- ization and rectification to separate the ferrous chloride, ing technologies for hydrochloric acid, and water from each other. In the rectifi- Table 1. Analysis of spent acid cation step the acid is concentrated and water passes through bath. for condensation, collection, and reuse in the rinse tank. Azeotropic HCl (17– 22%) is possible with this Concentrate Acid Sorp�on Diffusion Dialysis Evapora�ve Recovery technology. (waste) The scope of the fol- Generated (kg/hr) % (kg/hr) % (kg/hr) % lowing analysis is limit- HCl 0.5 0.3% 1.1 0.5% 0.75 1.1% ed to waste hydrochloric acid from typical batch/ FeCl 2 13.95 4% 23.75 5.8% 31.25 40.8% continuous pickling. H 2 O 172.75 95.7% 193.4 94.7% 44.5 58.1% This paper does not dis- cuss other chemical con- Total (kg/hr) 187.2 218.25 76.5 figurations or concentra- Acid Sorp�on Diffusion Dialysis Evapora�ve Recovery tions, nor does it discuss Return alternative configurations Acid (kg/hr) % (kg/hr) % (kg/hr) % of the three stated sepa- ration technologies. Total HCl 12 8% 11.4 6.2% 11.25 17.5% cost estimates are based FeCl 2 17.3 6.9% 7.5 2.2% 0 on primary contributing H 2 O 104 84.1% 164.85 91.6% 52.75 82.5% factors to capital, oper- ating, and maintenance Total (kg/hr) 133.3 @ 8% 183.75 @ 6% 64 @ 17% expenses. Table 2. Mass balance. 80 | WIRE JOURNAL INTERNATIONAL
TECHNICAL PAPERS What’s noteworthy in this paper WJI: Which technology best fits HCl on a very large scale. Due the wire industry? to the large capital and operating J&B Cullivan: Evaluation of expense, pyrohydrolysis was not recovery technologies should be covered in this paper. Another based on capital cost, waste or hybrid technology based on evap- co-product handling, impact on pro- oration is under development, but duction, and return on investment. commercialization is still a few Evaporation has several advantages years away. over the alternatives, but the Acid Jared and Bryan Cullivan Sorption producer has added better WJI: Do you find some people filtration and automated chemical reluctant to make improvements acid recovery evolved over the analyzers to address some problem because of the fear of the unknown? last three decades, and we see points. The evaporator's recovered J&B Cullivan: As more steel a similar trend with hydrochlo- acid is near the azeotrope (18%) pickling plants successfully install ric acid recovery technologies. and free of impurities, giving and operate acid recovery, the fear Today, very few sulfuric acid production people a consistent factor subsides. All of the recovery pickle houses run without acid source of quality replacement acid. methods addressed have overcome recovery. We expect the future However, steam is required for the many of their initial weak points of HCl and also mixed acids evaporation process. over the iterations of the products. will follow that trend. Sorption systems have added bet- WJI: Are there other recovery ter filtration equipment and online Questions for the authors? methods than those discussed here? analytics. The evaporative process They can be contact at J&B Cullivan: Yes, pyrohydroly- now operates at a lower temperature sales@betacontrol.com. sis is commonly used to regenerate and has a smaller footprint. Sulfuric Data and chemical analysis Sorption provides a better return acid in terms of concen- tration, 8%, but does not remove the ferrous chloride as Analysis is based on a typical wire pickling operation with effectively as the other technologies. Only 45.2% of the a spent acid bath as follows: five metric tons of spent pickle total ferrous chloride is rejected as concentrate/by-product. liquor per day (5,000 kg/day); 8% iron (by weight), 6% HCl Evaporative Recovery returns acid at a concentration (by weight). near the azeotrope (in this case 17.5%) and reduces the Mass balance comparison of the three different technologies concentrate/by-product mass by 63%, as compared with reveals advantages in the categories of acid recovery, metals only 10% for Sorption and an actual 5% increase in mass rejection, concentrate reduction, and acid concentration. for DD. In the absence of foreign contaminants that would Acid Sorption (Sorption) and Diffusion Dialysis (DD), affect the solubility (ex: zinc, chromium), ferrous chloride unlike evaporative recovery, are not as energy intensive will begin to form a crystal when the iron concentration and have fewer components (Table 4). Literature on exceeds a saturation point in an evaporative recovery Sorption and DD reveals high percentage returns on the system. Crystallized ferrous chloride (tetrahydrate) is amount of hydrochloric acid returned (not regenerated) sometimes preferred as a co-product because of the lower from the spent acid stream: 80-90% for Sorption (Cushnie 5 , p. 246) and 80-95% for DD (Cushnie 5 , p. 276). The mass balance on the spent pickle liquor Acid Diffusion Evapora�ve showed acid recovery rates of 84.8% and 91.2%, U�li�es respectively. Although the recovery rate of acid Sorp�on Dialysis Recovery is high, the quality of the acid is low (8% and Electricity (kWh) ~4 (est.) ~4 (est.) 6.34 6.2%, see Table 2). While DD has less than Water (L/hr) 112.2 194 ~5 (est.) half the contamination of ferrous chloride in its return acid, the acid concentration is often too Natural Gas 0 0 0.355 low to be returned directly to the pickle tank and (MMBtu/hr) requires additional concentration through evapo- ration due to the high volume (Cushnie 5 , p. 278). Table 3. Utility comparison for three methods. MARCH 2016 | 81
Recommend
More recommend