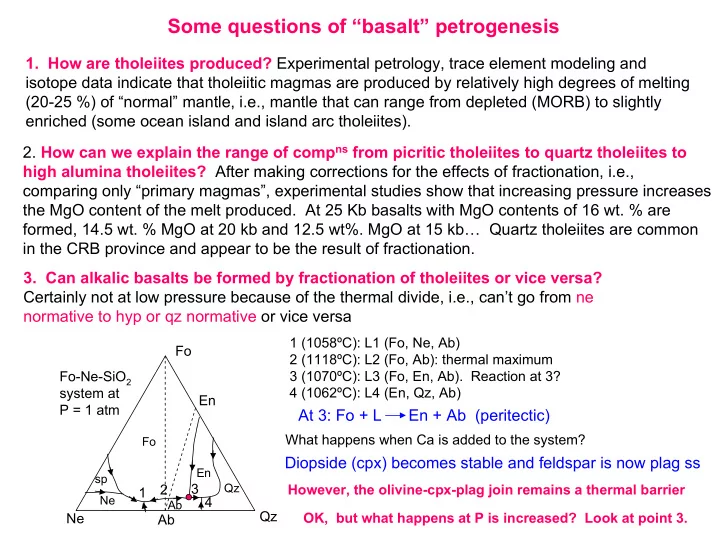

Some questions of “basalt” petrogenesis 1. How are tholeiites produced? Experimental petrology, trace element modeling and isotope data indicate that tholeiitic magmas are produced by relatively high degrees of melting (20-25 %) of “normal” mantle, i.e., mantle that can range from depleted (MORB) to slightly enriched (some ocean island and island arc tholeiites). 2. How can we explain the range of comp ns from picritic tholeiites to quartz tholeiites to high alumina tholeiites? After making corrections for the effects of fractionation, i.e., comparing only “primary magmas”, experimental studies show that increasing pressure increases the MgO content of the melt produced. At 25 Kb basalts with MgO contents of 16 wt. % are formed, 14.5 wt. % MgO at 20 kb and 12.5 wt%. MgO at 15 kb… Quartz tholeiites are common in the CRB province and appear to be the result of fractionation. 3. Can alkalic basalts be formed by fractionation of tholeiites or vice versa? Certainly not at low pressure because of the thermal divide, i.e., can’t go from ne normative to hyp or qz normative or vice versa 1 (1058ºC): L1 (Fo, Ne, Ab) Fo 2 (1118ºC): L2 (Fo, Ab): thermal maximum Fo-Ne-SiO 2 3 (1070ºC): L3 (Fo, En, Ab). Reaction at 3? 4 (1062ºC): L4 (En, Qz, Ab) system at En P = 1 atm At 3: Fo + L En + Ab (peritectic) What happens when Ca is added to the system? Fo Diopside (cpx) becomes stable and feldspar is now plag ss En sp 2 3 Qz However, the olivine-cpx-plag join remains a thermal barrier 1 Ne 4 Ab Qz Ne OK, but what happens at P is increased? Look at point 3. Ab
Basalt petrogenesis (cont.) Somewhere between 1 atm and 1 GPa (10 Kb) the invariant reaction equilibrium Effect of pressure crosses the En-Ab join and then the Fo-Ab (dry) on the Fo-Ne- join. So what? SiO 2 system The Fo-Ab join is no longer a thermal barrier so, in theory, at higher pressure subalkalic melts could fractionate to alkalic melts. As P is increased further, what happens? Liquids in equilibrium with Fo (Ol) –En (Opx) Fo –Ab (Plag or Spinel/Gnt at higher P) become En increasingly alkalic (ne normative) Tentative conclusions: (1) melting of mantle at higher pressures favors the formation of alkalic basalts (2) melting at low P favors melts richer in SiO 2 CO 2 at 20 Kb “dry” at 20 Kb What is the effect of adding volatiles to this system? H 2 O at 20 Kb Addition of H 2 O tends to produce more silica-rich basalts Addition of CO 2 tends to produce more alkali basalts.
Basalt petrogenesis (cont) As discussed earlier, pressure also has an effect on the MgO content of partial melts. Di We can illustrate this in the Fo-Di-SiO 2 system (homework problem system). As P increases the (pseudo) invariant point moves towards the Fo corner. Di Blue: L (Fo, Di ss , En ss ) at P = 1 atm Red: L (Fo, Di ss , En ss ) at P = 3 GPa En Fo Some general conclusions: Fo Qz En •Smaller degrees of partial melting favor the formation of alkalic basalts because Na behaves primarily as an incompatible element (D Na <1). At P = 1,2, or 3 GPa small degree partial melts are alkalic while larger degree partial melts are tholeiitic or picritic. •Higher pressure tends to favor the formation of alkalic melts •Higher pressure also tends to favor the formation of MgO rich melts (alkalic or subalkalic) •Melting of metasomatized mantle tends to favor the formation of alkalic basalt melts These are fairly robust general conclusions but they also represent a simplification of the actual situation because they are based on results from simple systems. We need to consider several additional factors: (1) inhomogeneous mantle sources, (2) variable degrees of partial meting, (3) trace element constraints, (4) isotopic constraints, (5) effect of volatiles (H 2 O and/or CO 2 )
Basalt petrogenesis (cont) Trace elements provide a test of fractionation models. For example, is it likely (or even possible) to derive alkalic basalts from a subalkalic parent? We concluded that it’s at least theoretically possible at higher pressures. Let’s assume that the data presented below represent magmas that we think might be related to each other by crystal fractionation. Could an alkalic OIB be derived from a tholeiitic MORB. As an example, let’s use the Rayleigh equation to model Rb. Reasonable value for D Rb for MORB phenocryst assemblages is ~0.03. For a Rb increase from ~2 to ~90, F L ~ 2%. In other words, ~98% crystallization required. L C 98% crystallization of a parental basalt would produce a − ( D 1 ) = i F i highly evolved liquid with extreme Fe-enrichment. It is L O C possible, in principle, to produce such a liquid but this i would not be an alkali basalt. Rayleigh equation
Basalt petrogenesis (cont) Q: If tholeiitic basalts and alkalic basalts are not related via fractionation at any pressure, could they be derived by different degrees of partial melting of a common mantle source? A: Perhaps, but each case must be considered on its own merits. First requirement would be that they have identical initial Sr, Nd and Pb isotopic ratios (assuming no assimilation). Second, major elements must be consistent with experimental petrology results. Third, trace elements must also be consistent. Example: Is it possible that the OIB and MORB shown on previous slide could be derived from a common mantle source? MORB rare earth element pattern clearly shows that MORB mantle source was depleted. Why? Careful trace element modeling could tell us if it was possible to derive the OIB from the same depleted source. To do this one would have to (1) assume a melting model, (2) know all the relevant distribution coefficients, (3) decide what pressures to use in the model, (4) know (or assume) the stoichiometry of the melting reaction… Quick feasibility test: Can we model La abundances? L C 1 Using the batch melting equation, and assuming D La ~ = i 0.01, we can reproduce the ratio of La in OIB to that in O − + C ( F ( 1 D ) D ) i i i L MORB (~12) with ~20% melting to produce MORB and 1% melting to produce OIB. Is this reasonable? More detailed modeling involving a variety of trace elements might provide a more definitive answer. However, many petrologists would argue that it is difficult (maybe impossible) to segregate such a small melt fraction from its crystalline residue.
Basalt petrogenesis [summary of comprehensive model proposed by Green and Ringwood (1967)] Valiant effort but this model, which was developed before we had abundant trace element and isotopic data, has problems.
Basalt petrogenesis (cont) While it seems possible, at least in principle, to get a wide range of basalt types from a uniform mantle source over a range of P, T, and melt fractions, we already know from xenoliths that the mantle is inhomogeneous, albeit over a fairly narrow major element compositional interval. We have fertile (?primordial) mantle, depleted mantle and depleted mantle later enriched. In a series of classic experiments, Jaques and Green (1980) carried out extensive high P/T experiments on depleted and enriched peridotites. Results: (1) Tholeiite: ~10 to ~30% partial melting with more silica-rich types at lower P (2) Difficult (maybe even impossible) to get alkalic basalt from a depleted mantle source Caveats: 1. It’s the depth (pressure) of magma segregation from its crystalline residue that is the important parameter. 2. Experiments cited assume some sort of equilibrium (batch) melting process. Recent work indicate that fractional melting may be the norm. Partial melting experiments on depleted lherzolites. Dashed lines represent percent partial melt produced. Curved lines show normative olivine content of the melt. “Opx out” and “Cpx out” represent the T&P at which these phases are completely melted. After Jaques and Green (1980). CMP 73, 287-310.
Basalt petrogenesis (cont.) Partial melting experiments on enriched (metasomatized) lherzolites Results: (1) Tholeiites are generated over a wider pressure range than in the case of depleted lherzolite (2) Alkalic basalts and basanites are generated over a relatively limited range of pressures and at significantly lower degrees of partial melting (near solidus melts). 900 pound gorilla: What is the influence of volatiles on mantle melting and how do we produce weird magmas like kimberlites, alnoites, carbonatites, lamproites, to name just a few. Partial melting experiments on enriched lherzolites. Dashed lines represent percent partial melt produced. Curved lines show normative olivine content of the melt. “Opx out” and “Cpx out” represent the T&P at which these phases are completely melted. Shaded field outlines PT conditions for alkali basalt generation. After Jaques and Green (1980). CMP 73, 287-310.
Recommend
More recommend