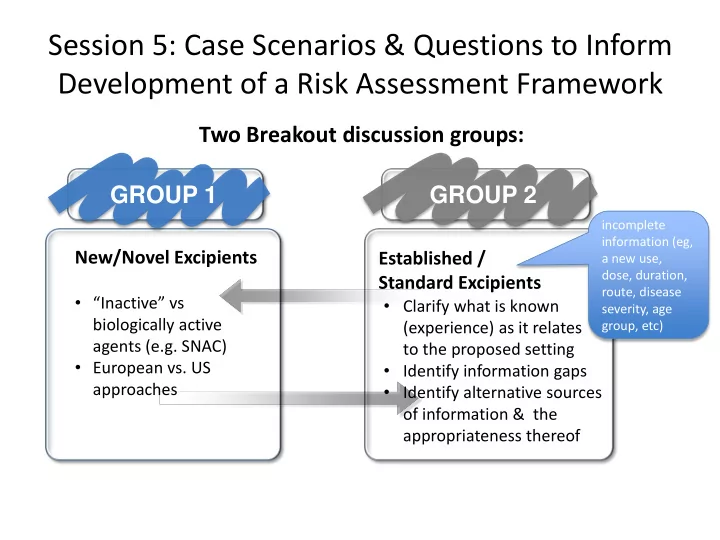

Session 5: Case Scenarios & Questions to Inform Development of a Risk Assessment Framework Two Breakout discussion groups: GROUP 1 GROUP 2 incomplete information (eg, New/Novel Excipients Established / a new use, dose, duration, Standard Excipients route, disease • “Inactive” vs • Clarify what is known severity, age biologically active (experience) as it relates group, etc) agents (e.g. SNAC) to the proposed setting • European vs. US • Identify information gaps approaches • Identify alternative sources of information & the appropriateness thereof
Breakout session discussion How to justify excipient use (novel, established) in paediatrics? What are the hurdles? Risk assessment & Information needs Information sharing platform • Can a common template or approach • Where to find the existing information? (framework) be developed for implementing • Platform to share information? risk assessments for individual excipients? (eg, STEP database) • What minimum information is required? • extending the FDA inactive ingredient What additional data is required? database to paediatrics • What circumstances and factors should be considered regarding the justification for Proposed Framework – juvenile tox studies ? Your opinion matters!! • Should toxicology studies with the final formulation be conducted? If so, when & • Would the proposed framework help address the issues of use of excipients in which studies ? paediatrics? • What alternative options are available if no additional information is available? • What are the pros and cons of the • What clinical trial design factors can be presented framework ? incorporated to provide information on the • What additional elements would you consider in the framework? safety of excipients? • Can we evaluate data on excipients & • Where are the knowledge gaps and how would you prioritize studies needed to approach the present in a format which will satisfy evaluation of excipients for paediatrics? regulators? 6/24/2016 Pediatric Excipients Workshop - Session 5 2
Breakout Deliverable: Proposed Framework to Assess Safety; Needs, Possible Solutions Consider 1. Is the use of the excipient justified? relevance to 2. Is there an established regulatory guideline or opinion? intended Age, 3. Is there relevant prior human use? (pharma, food) Route, Dose, 4. Is there other supporting information? Duration, Clinical – Adult, Paediatric, PK, PD, Modeling • Disease, etc … Nonclinical – Toxicology, PK/PD • What are the Similarity to other excipients? • Gaps? 5. Trials/Studies to fill the gaps Design, Value • Better info • What are the pros and cons of the presented framework? sharing? • What additional elements would you consider in the framework? 6/24/2016 Pediatric Excipients Workshop - Session 5 3
Recommend
More recommend