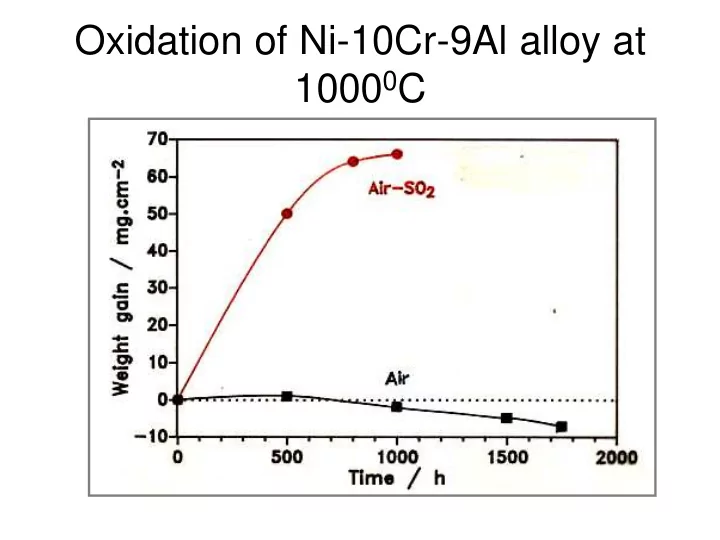

Oxidation of Ni-10Cr-9Al alloy at 1000 0 C
Pressure Dependence of SO 2 • Reaction with SO 2 is tempertaure and Pressure Dependent • At 1 atms. SO 2 pressure at 500-600 o C 7Ni + SO 2 = 4NiO + Ni 3 S 2 ------- [1] At this pressure as per Phase stability Diagram, NiSO4 is also stable, because of which there is additional reaction 9Ni + NiSO 4 = 8NiO + Ni 3 S 2 ------- [2] Because of which the rate of reaction is very fast. As the pressure is reduced from 1 atm to 0.1 atms. NiSO 4 is no longer stable and reaction 2 does not occur and rate falls.
Phase Stability Diagram Of Ni
Temperature Dependence of SO 2 • NiSO 4 is stable upto 600 o C, hence no possibility of reaction [2]. • At 635 o C Ni-S liquid starts becoming stable. The reaction from 700-900 o C are linear and rates are proportional to SO 2 pressure. • The rate decreases further with increase in temp. above 800 o C because of instability of N i3 S 2 above 800 o C. • The sulphide formation becomes thermodynamically unstable at high temperatures and at low SO 2 pressure • Under these conditions, the corrosion product is is only NiO and no sulphide thus corrosion rate falls drastically. • That is why rate of corrosion of Ni in SO 2 at 1000 o C is almost same as rate of oxidation of Ni.
Sulphidation of Ni Vs Co
Sulphidation of Ni in SO 2 + O 2 SO 2 (g) + O 2 (g) = SO 3 (g) NiO (s) + SO 3 (g) = NiSO 4 (s)
Oxidation of Binary Alloys of Ni-Cr
Sulphidation of Fe-Cr, Ni-Cr and Co-Cr Alloys
Effect of Pre-Oxidation on Sulphidation
Mechanism of SO 2 /SO 3 Reaction • Reaction starts with the sulphide formation of most stable element and further growth occurs mainly by lattice diffusion of ions thru sulphide scales. • Formation of mixed oxide scale occurs even when the partial pressure of oxygen or sulphur is dominant at one particular point. – this happens because of continuous increase in conc. Of other gas below the main scale interface. • How the sulphur partial pressure in craeses below oxide scale (a) Dissolution of SO 2 – not sound as s disolution is very low. (b) Gas dissolution thru’ pores and cracks and hence establishes con. Gradient. (c )Direct reaction of SO2 which can produce S C + SO 2 = S + CO 2
Recommend
More recommend