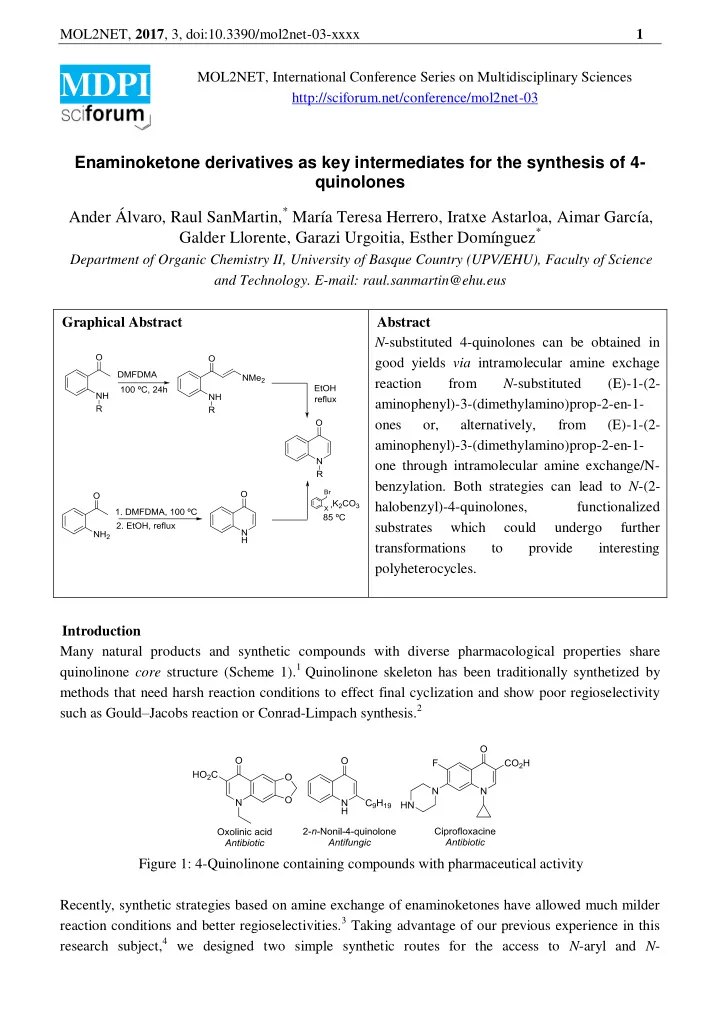

MOL2NET, 2017 , 3, doi:10.3390/mol2net-03-xxxx 1 MOL2NET, International Conference Series on Multidisciplinary Sciences MDPI http://sciforum.net/conference/mol2net-03 Enaminoketone derivatives as key intermediates for the synthesis of 4- quinolones Ander Álvaro, Raul SanMartin, * María Teresa Herrero, Iratxe Astarloa, Aimar García, Galder Llorente, Garazi Urgoitia, Esther Domínguez * Department of Organic Chemistry II, University of Basque Country (UPV/EHU), Faculty of Science and Technology. E-mail: raul.sanmartin@ehu.eus Graphical Abstract Abstract N -substituted 4-quinolones can be obtained in good yields via intramolecular amine exchage reaction from N -substituted (E)-1-(2- aminophenyl)-3-(dimethylamino)prop-2-en-1- ones or, alternatively, from (E)-1-(2- aminophenyl)-3-(dimethylamino)prop-2-en-1- one through intramolecular amine exchange/N- benzylation. Both strategies can lead to N -(2- halobenzyl)-4-quinolones, functionalized substrates which could undergo further transformations to provide interesting polyheterocycles. Introduction Many natural products and synthetic compounds with diverse pharmacological properties share quinolinone core structure (Scheme 1). 1 Quinolinone skeleton has been traditionally synthetized by methods that need harsh reaction conditions to effect final cyclization and show poor regioselectivity such as Gould – Jacobs reaction or Conrad-Limpach synthesis. 2 Figure 1: 4-Quinolinone containing compounds with pharmaceutical activity Recently, synthetic strategies based on amine exchange of enaminoketones have allowed much milder reaction conditions and better regioselectivities. 3 Taking advantage of our previous experience in this research subject, 4 we designed two simple synthetic routes for the access to N -aryl and N -
MOL2NET, 2017 , 3, doi:10.3390/mol2net-03-xxxx 2 benzylsubstituted 4-quinolones, potential precursors of more complex polyheterocyclic systems (Scheme 1). Both strategies were based on an amine exchange reaction, however, while in route A, the quinolinone ring would be provided by intramolecular N -arylation of the intermediate enaminoketone, in route B, the intramolecular amine exchange reaction would effect the cyclization itself. Scheme 1. Designed pathways to synthesize 4-quinolones from 1-aril-3-dimetilamino-2-propen-1-ones Results and Discussion We began our research by testing designed route A. After synthetizing required enaminoketone 1 by reaction of 2-bromophenylacetophenone with N,N -dimethylformamide dimethyl acetal (DMFDMA), a number of reaction conditions was assayed in order to achieve the amine exchange/intramolecular N - arylation tandem sequence that would provide the desired 1-substituted-4-quinolinone (Table 1). Different palladium (0) and palladium (II) sources were tested in combination with phosphine-type quelating ligands , commonly used in N -arylation reactions, such as BINAP or DPPF. Regarding the base, we used NaO t Bu, Cs 2 CO 3 or K 3 PO 4 and aniline and benzylamine were the amines of choice in these assays. Table 1. Selected assays for the reaction of dimethylamino-2-propen-1-one 1 with amines 2 3a (%) a 3b (%) a Reaction conditions Entry 1.0 eq. 1 , 1.4 eq. 2 , 1.6 eq. K 2 CO 3 , 1.6 eq. NaO t Bu, 10% Pd(PPh 3 ) 4 , 3 ml/mmol Tol, ,2h 1 traces 30% 1.0 eq. 1 , 1.4 eq. 2 , 0.08 eq. DPPF, 1.6 eq. NaO t Bu, 6% Pd(dba) 3 , 3 ml/mmol Tol, 24h 2 20% 20% 3 1.0 eq. 1 , 2.0 eq. 2 , 1.6 eq. K 3 PO 4 , 0.08 eq. BINAP, 5% Pd(OAc) 2 , 10 ml/mmol Tol, 4 10% 13% ml/mmol H 2 O, 5h 4 1.0 eq. 1 , 2.0 eq. 2 , 1.6 eq. K 3 PO 4 , 0.08 eq. BINAP, 5% Pd(OAc) 2 , 10 ml/mmol Tol, 22h 27% 12% a Yield calculated by 1 H NMR employing 3,4,5-tricholoropyridine as internal standard. Unfortunately, only in few occasions 4-quinolinone was obtained and, in all cases, in poor yield (Table 1). The use of Pd(PPh 3 ) 4 as palladium source and a combination of K 2 CO 3 and NaO t Bu provided the
MOL2NET, 2017 , 3, doi:10.3390/mol2net-03-xxxx 3 N -phenyl-4-quinolinone 3b in 30% yield. On the other hand, the best result for the N -benzyl-4- quinolinone 3a was obtained when Pd(OAc) 2 was used in presence of BINAP and toluene as reaction medium. In order to improve the yields we tested the stepwise version but, unfortunately, in no case was the 4-quinolinone detected (Scheme 2). Scheme 2. Stepwise synthesis of 4-quinolinones fron enaminoketone 1 Considering the poor results obtained, we decided to focus our efforts on the route B (Scheme 1). As usual, required enaminoketones were prepared from the corresponding 2-aminophenylacetophenone by reaction with DMFDMA. Surprisingly, in the case of N -phenylamine, the reaction with DMFDMA provided 1-phenylquinolin-4(1H)-one 3b in an excellent yield. In the rest of the cases, the corresponding 4-quinolinone was obtained by refluxing in toluene. 4-quinolone 3d was prepared in excellent overall yield from 2-aminoacetophenone. In the case of N -benzylaminofenil enaminoketone 3a and N -phenyl enaminoketone 3c , the corresponding quinolinones were obtained in lower overall yields, 69% and 41%, respectively. Scheme 3. Synthesis of 1-substituted-4-quinolinones reaction from N-substituted enaminoketones 6 by intramolecular amine exchange With the aim of improving global yields and, taking into account that the substituent in the nitrogen of the aniline precursor 6 seemed to have a negative effect on the intramolecular amine exchange reaction, we decided to introduce said substituent after carrying out the cyclization. (Scheme 4). The aminomethynelation/amine exchage/N-alkylation sequence allowed us to improve the previous results and we obtained quinolinones 4a and 4c in 74% overall yield.
MOL2NET, 2017 , 3, doi:10.3390/mol2net-03-xxxx 4 Scheme 4.Synthesis of quinolinones 3a and 3c by N-alkylation of 4-quinolinone 3d Conclusions The sequence amine exchange/intramolecular N -arylation has provided the N-substituted 4-quinolones with modest yields so far and, therefore, further research is needed in order to improve the results. On the other hand, N-substituted 4-quinolones can be obtained in good yields via intramolecular amine exchage reaction from N-substituted (E)-1-(2-aminophenyl)-3-(dimethylamino)prop-2-en-1- ones or, alternatively, from (E)-1-(2-aminophenyl)-3-(dimethylamino)prop-2-en-1-one via intramolecular amine exchange/N-benzylation. Both strategies can lead to N -(2-halobenzyl)-4- quinolones, functionalizated substrates which could undergo further transformations to provide interesting polyheterocycles. References 1. a) Okamoto, N. ; Takeda, K.; Ishikura, M.; Yanada, J. Org. Chem. 2011 , 76 , 9139. b) Dhar, T. G. M.; Watterson, S. H.; Chen, P.; Shen, Z.; Gu, H. H. Norris, D.; et al. J . Bioorg. Med. Chem. Lett ., 2003 , 13 , 547. 2. See, for example: a) Bunce, R.; Nammalwar, B. Org. Prep. Proced. Int . 2010 , 42 , 557. b) G. Hiltensperger, N. G. Jones, S. Niedermeier, A. Stich, M. Kaiser, J. Jung, et al. J. Med. Chem. 2012, 55, 2538. c) Joule, J. A.; Mills, K. Het. Chem. 2000 , 133. 3. See for example: a) Wang, F.; Jin, L.; Kong, L.; Li, X. Org. Lett. 2017 , 19 , 1812. B) Tois, J.; Vahermo, M.; Koskinen, A. Tetrahedron Lett . 2005 , 46 , 735. 4. a) Hernández, S.; Moreno, I.; SanMartin, R.; Herrero, M.T.; Domínguez, E. Org. Biomol. Chem. 2011, 9, 2251. b) Hernández, S.; Moreno, I.; SanMartin, R.; Gómez, G.; Herrero, M.T.; Domínguez, E. J. Org. Chem. 2010, 75, 434. Acknowledgments We thank Basque Government (IT-774-13) and the Spanish Ministry of Economy and Competitiveness (CTQ2013-46970-P) for financial support. Finally, technical and human support provided by SGIker of UPV/EHU is gratefully acknowledged..
Recommend
More recommend