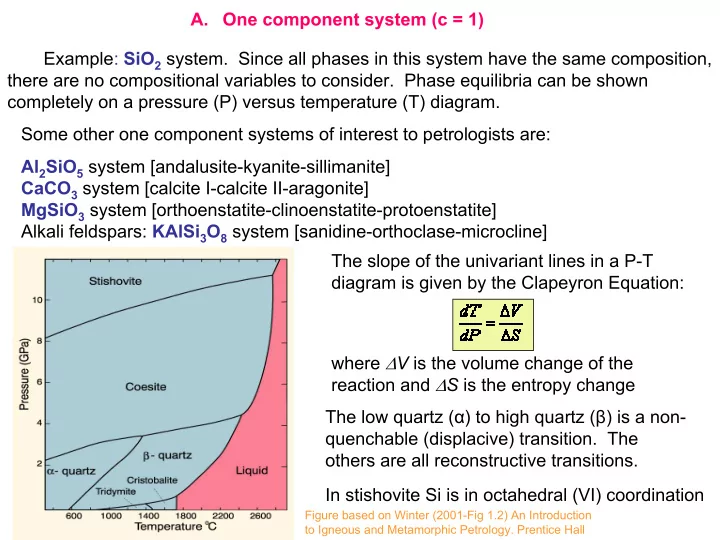

A. One component system (c = 1) Example: SiO 2 system. Since all phases in this system have the same composition, there are no compositional variables to consider. Phase equilibria can be shown completely on a pressure (P) versus temperature (T) diagram. Some other one component systems of interest to petrologists are: Al 2 SiO 5 system [andalusite-kyanite-sillimanite] CaCO 3 system [calcite I-calcite II-aragonite] MgSiO 3 system [orthoenstatite-clinoenstatite-protoenstatite] Alkali feldspars: KAlSi 3 O 8 system [sanidine-orthoclase-microcline] The slope of the univariant lines in a P-T diagram is given by the Clapeyron Equation: where ∆ V is the volume change of the reaction and ∆ S is the entropy change The low quartz ( α ) to high quartz ( β ) is a non- quenchable (displacive) transition. The others are all reconstructive transitions. In stishovite Si is in octahedral (VI) coordination Figure based on Winter (2001-Fig 1.2) An Introduction to Igneous and Metamorphic Petrology. Prentice Hall
Stability fields for Al 2 SiO 5 polymorphs 10 Insets show the change in entropy (S) as a function of T along isobaric path 1 and the change in volume (V) 9 along isothermal path 2 (note discontinuities In S and V) 8 Path 2 7 6 P (kbars) S K V 5 S 4 T 3 A P Path 1 2 1 0 0 200 400 600 800 1000 T (deg C)
B. Two component systems (binary systems): common types [1] eutectic system, e.g., Diopside (Di)--Anorthite (An) in which there is little or no solid solution between the end member mineral components [2] peritectic system (with intermediate compound), e.g., Forsterite (Fo)--Silica (SiO 2 ), in which the intermediate compound Enstatite (En) melts incongruently [3] “Double” eutectic system, e.g. Nepheline (Ne)--Silica (SiO 2 ) in which the intermediate compound (Ab) melts congruently. [4] system showing complete miscibility in both liquid and solid phases, e.g., Forsterite (Fo)—Fayalite (Fa) and Albite (Ab)—Anorthite (An) [5] system with solvus , e.g., Albite (Ab)—Orthoclase (Or): Enstatite (En)–Diopside (Di). Such systems exhibit variable solid solution between the end member minerals. [6] System with H 2 O as a component, e.g., Albite (Ab)—H 2 O, Quartz—H 2 O In the first five cases, it is convenient at first to express the equilibria at constant pressure; the phase diagram is referred to as a (TX) P section. In the case where one variable is held constant, the phase rule becomes: f = c - p + 1 . Compositional variations are expressed as weight % although we could have expressed the composition in mole %, or in oxygen units.
Diopside (CaMgSi 2 O 6 )-anorthite (CaAl 2 Si 2 O 8 ) system (c = 2) P = 1 atm b a solidus 1. Discuss: Cooling of bulk compositions “a” starting at 1450ºC, comp “b” starting at 1600ºC 2. Discuss: Melting of compositions “a” and “b” starting at a temperature below the solidus Concepts: Invariant, univariant and divariant assemblages Equilibrium crystallization and fractional crystallization Equilibrium melting and fractional melting Lever Rule, tie lines Figure from Winter (2001) An Effect of pressure, effect of addition of water to system Introduction to Igneous and Metamorphic Petrology.
Examples of crystallization of pyroxene and plagioclase pyroxene Plag In this photomicrograph of a Mount Baker In this photomicrograph of a troctolite andesite, we would interpret the texture as from the Stillwater Complex, we would indicating that plagioclase crystallized at interpret the texture as indicating that the same time as augite—both are olivine crystallized before plagioclase. euhedral. The texture is “porphyritic.” “Cumulate” texture.
Diopside (CaMgSi 2 O 6 )-anorthite (CaAl 2 Si 2 O 8 ) system (c = 2): effect of P 1 Eutectic composition P (Gpa) 0.5 0 40 50 60 An % Note: The effect of increasing pressure is (1) increase the liquidus and solidus T, particularly for Diopside; (2) Change the composition of the eutectic.
Diopside (CaMgSi 2 O 6 )-anorthite (CaAl 2 Si 2 O 8 ) system (c = 2): effect of H 2 O 1100 1300 1500 1 1270 1520 1125 1570 P = P H2O (Gpa) An L Di L Di + V L L An + V 0 1560 1391 T(ºC) T(ºC) Note: Effect of H 2 O is to: 1. Lower melting T dramatically 2. Effect of H 2 O is much more pronounced in An than in Di, i.e, more polymerized melts show stronger effect. 3. Dramatic shift in composition of the eutectic with H 2 O saturated conditions. 4. Reduced stability of plagioclase with increasing f H2O
Binary systems (c = 2) showing complete miscibility in liquid and solid phases Albite (Ab)—Anorthite (An) Forsterite (Fo)—Fayalite (Fa) P = 1 atm P = 1 atm xal L L xal xal L xal L X X X X X X X X T, P Variables: T, P Ab Ab An Fa Fo Fo An Fa Phase Rule: f = c – p + 1 = 3 - p Phase Rule: f = c – p + 1 = 3 - p Above liquidus: f = ?, Below solidus, f = ? Above liquidus: f = ?, Below solidus, f = ? Between liquidus and solidus, f = ? Between liquidus and solidus, f = ?
Equilibrium crystallization/melting of composition An 60 1500ºC: a=liq, p=1, f =2 1480ºC: liq=b: xals=c, p=2, f=1 1450ºC: liq=d: xals=c: p=2, f=1 Lever Rule: d f e Xals Liq Liq = ef Xals de Tie lines, e.g., b-c, d-f, g-h: Figures from Winter (2001) An 1340ºC: liq=g: xals=h: p=2, f=1 <1340ºC: xals=i (An 60 ), p=1, f=2 Introduction to Igneous and Metamorphic Petrology.
Recommend
More recommend