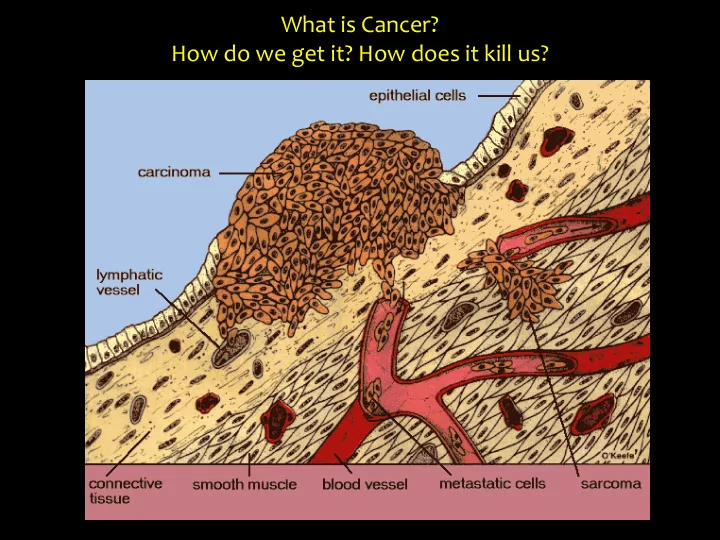

What is Cancer? How do we get it? How does it kill us?
Two distinct cancer research themes: 1. tumor onset (how does it all start? Is it really just a question of bad luck?) 2. metastasis and phenotypic plasticity (what has personalized medicine to do with that?)
Mythbusting Cancer Research `For every complicated problem there is a solution that is simple, direct, understandable, and wrong. H.L. Mencken
Colorectal Cancer: the second deadliest
Cancer: a multistep genetic process 3 rd hit 1 st hit 2 nd hit (gene C mutation) (gene A mutation) (gene B mutation) sequential clonal expansions (Darwin docet!)
Cancer: a multistep genetic process
The Adenoma-Carcinoma Sequence in Colorectal Cancer: a genetic paradigm for tumor initiation, progression, and metastasis intestinal carcinoma ACF epithelial cell adenoma SMAD2/4 p53 APC K-RAS chr. 18q LOH chr. 17p LOH What about the rate-limiting, initiating ( APC ) mutation event? Is it just a ‘random’ event (bad luck, shit happens), or do extrinsic (environmental) factors favor its occurrence?
Cancer: the role of “bad luck” vs. environmental risk factors
Cancer: the role of “bad luck” vs. environmental risk factors
Colorectal Cancer: the second deadliest, highest stem cell division rate (10 12 over 60 yrs), and yet largely due to environmental factors.
Cancer: the role of “bad luck” vs. environmental risk factors
Why do we get colon cancer? 4 main etiological factors: 1. Age 2. Diet (life style) 3. Inflammation 4. Genetics It’s all in the stem cell niche!
intestinal carcinoma epithelial cell ACF adenoma APC SMAD2/4 K-RAS p53 chr. 18q LOH chr. 17p LOH stem cell niche alterations (i.e. expansion of cell targets for tumor initiation and progression) Aging Western-style diet Predisposition IBD metaplasia (stem cell reprogramming)
Why do we get colon cancer? 4 main etiological factors: 1. Age 2. Diet (life style) 3. Inflammation 4. Genetics It’s all in the stem cell niche!
How to model Western-Style diet-induced colon cancer in the mouse?
How to model Western-Style diet-induced colon cancer in the mouse? 500% AIN76A 400% % of control diet NWD1 300% NWD2 200% 100% 0% Fat Vit D3 Ca2+ Fiber Methyl donors NWD1: - 25% of C57BL/6J mice develop colon tumors (n= 1-2); - Tumors arise at 1.5-2 years of age; - Ratio carcinoma-to-adenoma of ~10%; → First and only model of dietary induced sporadic CRC NWD2 : Increased Vit D3 and Ca 2+ rescue the tumorigenic effect
Inflammatory Bowel Diseases: Crohn’s and Ulcerative Colitis
Modelling inflammatory bowel disease by Dextran Sodium Sulfate (DSS) H 2 0 1 2 3 4 5 6 7 8 9 10 Lgr5-EGFP SACRIFICE 3% DSS H 2 0 1 2 3 4 5 6 7 8 9 10 Lgr5-EGFP SACRIFICE
The intestinal stem cell niche
The intestinal stem cell niche Lys creERT2 cKit + Paneth-like (J. Van Es, H. Clevers) cells (in colon) Lgr5 EGFP-IRES-creERT2 cKit creERT2 (N. Barker, H. Clevers) (D. Saur)
Both Western-style diet and inflammation suppress Lgr5 + stem cell function GFP/YFP GFP GFP/RFP GFP GFP Ki67 Control diet Control GFP GFP GFP/RFP GFP/YFP GFP Ki67 NWD1 diet 3% DSS → fewer Lgr5 + stem cells → Lgr5 + stem cells cease to proliferate → Lgr5 + stem cell lineage tracing is inhibited
NWD1 inhibits proliferation, lineage tracing, and tumor-initiating capacity of Lgr5 + stem cells through the vitamin D receptor.
Western-style dietary factors and in particular vitamin D depletion inhibit Lgr5 stem cell function The western-style NWD1 diet (low in vitamin D) represses the stem cell function of Lgr5 + cells and their capacity to underlie cell turnover in the small an large intestine. Are there other stem cells involved? Or are the dietary effects mediated by the niche?
‘Mini - Gut’ Organoid Assay Matrigel + growth factors small intestine crypts 500 crypts/ well or colon 5h 1day 5 days 0h
Intestinal stemness is increased by Western-style diet 5 day old organoids 5x 10x 20x 200 180 AIN76A number of organoids 160 140 120 100 80 60 NWD1 40 20 0 AIN76A NWD1 NWD2 → notwithstanding the fact that the Western-style diet suppresses NWD2 stemness of Lgr5 + cells, mini-gut organoid forming capacity is increased. Hence, a distinct stem cell type is activated upon Western-style diet.
The intestinal stem cell niche
Functional analysis of stem vs. niche effects: the organoid reconstitution assay exp. mouse A exp. mouse B Single cell digest of (e.g. control diet) (e.g. NWD1 diet) intestinal mucosa FACSort stem ( Lgr5 + ) and niche (Paneth) cells separately 1000 Lgr5 + CBCs 1000 Paneth cells from mouse A from mouse B optional: genetic (e.g. siRNA) or biochemical modification of sorted lineages 30’ co -incubation at 37 ° C Plate out in matrigel for Lgr5 cells ‘mini - gut’ assay Paneth cells
Regeneration Inflammation Paneth cells acquire stem cell properties Depletion of Lgr5 + stem cells and repopulate the intestinal epithelium. Lineage tracing Homeostasis Paneth cells provide niche support to Lgr5 + stem cells. +4 Lineage tracing Lgr5 + Lyz + Paneth +4 Lyz + Paneth Regeneration Western style diet Niche reinforcement by Paneth cells, Lgr5 + stem cells inactivation quiescent stem cell (+4) activation.
Overall, colon cancer risk factors such as diet and inflammation profoundly affect both niche and stem cells resulting in improved regeneration upon tissue injury. The recruitment of new stem cell types and the overall increased proliferation rate ultimately results in an increased mutation rate and cancer risk.
For every complicated problem there is a solution that is simple, direct, understandable, and wrong. H.L. Mencken
Two distinct cancer research themes: 1. tumor onset (how does it all start? Is it really just a question of bad luck?) 2. metastasis and phenotypic plasticity (what has personalized medicine to do with that?)
Tumorigenesis and Metastasis: Discontinous Evolution Distant Site Metastases 3 rd hit 2 nd hit 1 st hit Tumor Evolution at the Primary Site (epi)genetic mutation load ( Darwin docet : sequential clonal expansion) x . x . . . x . . . 3 rd hit 2 nd hit x 1 st hit x Dissemination (CTCs & DTCs) 20 30 40 50 60 70 x genetic hit surgery . (mutation) Age (years) epigenetic hit . EMT (red: EMT; yellow: MET)
Phenotypic Plasticity
The current ‘hype’ on personalized cancer therapy is misplaced and unjustified
2018 2002 ‘a large majority of driver gene mutations are common to all metastases’ ‘ the driver gene mutations that were not shared by all metastases are unlikely to have functional consequences ’
Som ome ea earl rly (70’s and 80’s) exp xperimental evid viden ence th that t th thin ings gs are not ot as sim imple e as th that: Tumor cells are capable of contributing to normal development and generate phenotypically normal chimeric organism with tumor cells present in most adult tissues ( mouse teratocarcinoma cells: Illmensee & Mintz, 1976; chicken embryonic cells infected with Rous sarcoma virus: Rous, 1979; Dolberg & Bissell, 1984; Stoker, Hatier, & Bissell, 1990). Mina Bissel, PhD: phenotype is dominant over genotype!! Notably, when cultured in vitro , these embryonic cells rapidly reveal pronounced tumorigenic features and undergo neoplastic transformation . Hence, the embryonic environment seems to attenuate oncogenesis possibly by inducing differentiation and suppressing cancer stemness.
Type, density and location of tumor-infiltrating immune cells in large cohorts of human colorectal cancers represent the strongest prognostic factors in terms of freedom from disease and overall survival at all stages of clinical disease (Galon et al., Science 2006). Jerome Galone, PhD
The Overhyping of Precision Medicine Science has a history of inflated promises when it comes to disease treatment . by Nathaniel Comfort , Dec. 16, 2016 www.theatlantic.com/health/archive/2016/12/the-peril-of-overhyping-precision-medicine/510326
Phenotypic Plasticity
The invasion-metastasis cascade: two main theories …. “seed and soil” (single CTCs) vs . “collective cell migration”
Epithelial-to-mesenchymal transition: not a B&W process Epithelial markers : E/M markers : Mesenchymal markers : • • E-cadherin Vimentin ?? • • Cytokeratin Fibronectin • • Desmoplakin N-cadherin • 𝛃 -SMA • Laminin Functional features : Functional features : Functional features : • • • Tight cell-cell adhesion Weak cell-cell No cell-cell adhesion • • Non-motile adhesion Motile • • • Non-invasive Metastable Invasive EMT transcription factors: ZEB1, ZEB2, Snail, Slug, Twist, FoxC2
Phenotypic Plasticity and Metastasis: conventional immortalized cell lines as experimental models
Common colon cancer cell lines encompass a CD44 high EpCAM low subpopulation with mesenchymal and highly motile and invasive features Andrea Sacchetti
CD44 high EpCAM low colon cancer cells express EMT markers and transcription factors ( ZEB1 )
Recommend
More recommend