
Relationship between pS 2 and pSO 2
HOT CORROSION
HOT CORROSION Metals and alloys experience accelerated oxidation / sulphidation when their surfaces are covered with a thin film of fused salt in an oxidizing gas atmosphere at elevated temperatures Types of Hot Corrosion • Temp. range 850-950 o C (m.p. • Temp. range 650-800 o C of Na 2 SO 4 884°C) • Formation of low melting • Initial attack on protective mixtures Na 2 SO 4 -NiSO 4 oxide film eutectics • Chromium depletion, • High partial pressure of SO 3 oxidation of the base material (g) – required accelerates • Typical pitting Type I Type II (HTHC) (LTHC)
HIGH TEMPERATURE HOT CORROSION Occurs at high temperature Salt deposit on metal / alloy in liquid state Very high corrosion rate Kinetics - mainly linear Corrosion of Ni in presence of Na 2 SO 4 at 900 o C Melting point of Na 2 SO 4 - 880 o C Ref: Introduction to High Temperature Oxidation and corrosion, A.S.Khanna, IIT Bombay, ASM International, 2002
LOW TEMPERATURE HOT CORROSION Reaction rate at beginning – slow till certain point – incubation period Later rate increases suddenly and follow linear kinetics Occurs at lower temperatures – lower than melting temperature of salt Initial stages - Na 2 SO 4 solid – corrosion reaction involves oxidation of Ni to NiO NiO + SO 2 = NiSO 4 Melting point of NiSO 4 - 671 o C Ref: Introduction to High Temperature Oxidation and corrosion, A.S.Khanna, IIT Bombay, ASM International, 2002
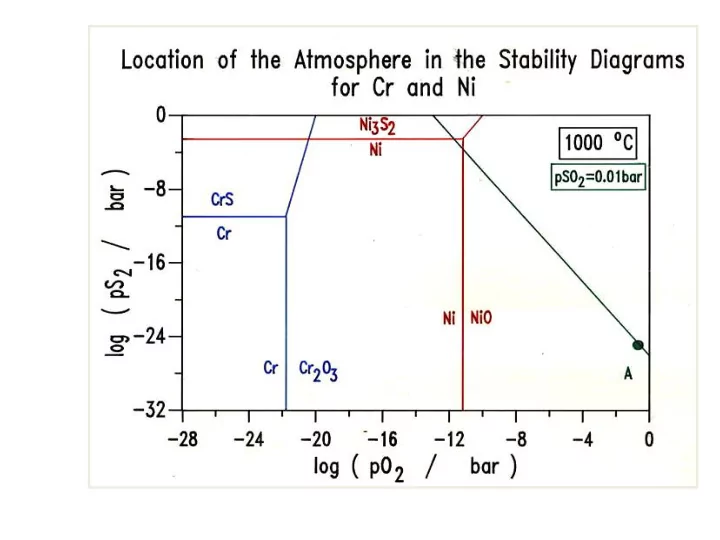
FLUXING MECHANISM OF HOT CORROSION Oxyanion sodium sulphate melt similar to acid-base chemistry of aqueous solutions Na 2 O + SO 3 = Na 2 SO 4 log K (1200 K) = -16.7 log aNa 2 O = melt basicity log aSO 3 = melt acidity Dissolution on NiO can be represented by the following equations: Basic dissolution : 2NiO + O 2- (Na 2 O) + ½ O 2 = 2NiO 2- (NaNiO 2 ) Acidic dissolution : NiO = Ni 2+ + O 2- Further O 2- + SO 3 = SO 4 2- (NiSO 4 ) Ref: Robert A. Rapp, Hot corrosion of materials: a fluxing mechanism? Corrosion Science 44 (2002) 209 ± 221
SUSTAINED ACIDIC DISSOLUTION Acidic Basic dissolution dissolution MO = M 2+ + O 2- 2MO + O 2- (Na 2 O) + ½ O 2 = Further O 2- + SO 3 = SO 4 2- 2MO 2- (NaNiO 2 ) (NiSO 4 ) melt gradually consumes the molten Na 2 SO 4 – solvent for NiSO 4 salt gets saturated with which reacts with nickel migrating NaNiO 2 outwards through the inner layer with a continuous sulphide network reaction subsides Na 2 SO 4 is not consumed and the reaction continues for extended periods of time
Isothermal Tests Cyclic Tets Schematic of Bead Test Crucible Test
Schematic of a Burner Rig
Hot Corrosion of Nickel
FORMATION OF EUTECTIC MIXTURE
Temperature dependence of the corrosion rate of Ni-30% Cr coated with Na 2 SO 4 (2.5 mg/cm 2 ) in latm. of O 2 + 1% (SO 2 + SO 3 ) and Ni-20% Cr (with no salt deposit) in 1 atm. of SO 2 : O2 : 1 : 1.
Na2SO4 induced hot corrosion of Ni-30% Cr and Co-30% Cr in O 2 + 1% (SO 2 + SO 3 ) and O 2 + 0.15% (SO 2 + SO 3 ) exposed for 24 h.
ROLE OF ALLOYING ELEMENTS Vanadium and molybdenum • Improves mechanical properties • Presence makes alloy highly susceptible to hot corrosion Titanium, aluminum, and niobium • increase the hot corrosion resistance.
Recommend
More recommend