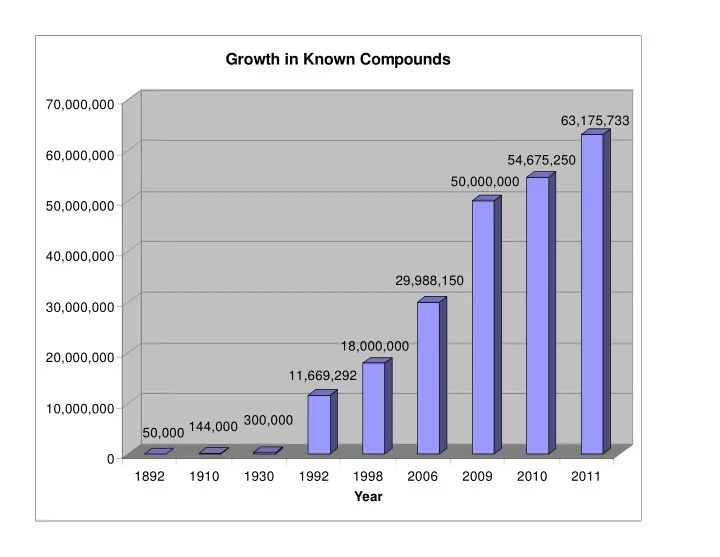

Growth in Known Compounds 70,000,000 63,175,733 60,000,000 54,675,250 50,000,000 50,000,000 40,000,000 29,988,150 30,000,000 18,000,000 20,000,000 11,669,292 10,000,000 50,000 144,000 300,000 0 1892 1910 1930 1992 1998 2006 2009 2010 2011 Year
There are many competing naming conventions:
Sometimes, the use of trivial names is particularly convenient:
IUPAC Nomenclature alkane: alk an e a multiplicative prefix ( # of C in parent chain) a functional group suffix a hybridization state suffix ( sp 3 , sp 2 , sp )
Generating names of isomeric alkanes: 1. Find the longest contiguous hydrocarbon chain in the molecule. It becomes the PARENT, or root, name. 2. Identify any substituent group replacing hydrogens on the parent chain. 3. Name each substituent group by derivation from its parent name: alkane - ane + yl = alkyl These alkyl groups will be used as prefixes to the parent name of the compound. 4. Locate all substituent group position on the parent chain by numbering the parent chain. These numbers are called LOCANTS. When there is a choice, assign substituents the lowest possible combination of locants determined by the first point of difference in locant. Hyphens always separate locants from letters in substituent names. Substituents are listed alphabetically as prefixes to the parent name.
5. Identical substituents are grouped and a multiplicative prefix is used: di, tri, tetra, penta, hexa, etc. 6. Complex substituents are encompassed by parentheses, or their common names are used.
Simple and complex alkyl substituents classification Primary (1 � ) Secondary (2 � ) H H H R C C C CH 3 H H H H H CH 3 C C R H CH 3 CH 3 R C CH 3 CH 3 Tertiary (3 � )
IUPAC and common names of constitutional isomers of the C 3 and C 4 alkyl substiutents substituent common name IUPAC name H H n -propyl propyl CH 3 C C R H H 1-methylethyl CH 3 isopropyl or H C R methylethyl CH 3 H H H n -butyl butyl H 3 C C C C R H H H sec -butyl 1-methylpropyl H H or CH 3 C C R s -butyl methylpropyl H CH 3 H H isobutyl 2-methylpropyl CH 3 C C R H 3 C H tert -butyl 1,1-dimethylethyl CH 3 or H 3 C C R t -butyl dimethylethyl CH 3
Some examples 4-(1-methylethyl)heptane 4-isopropylheptane 2,3-dimethyl-6-(2-methylpropyl)decane 6-isobutyl-2,3-dimethyldecane not 6-butyl-2,3,8-trimethylnonane not 5-isobutyl-2-isopropylnonane
Conformational analysis of butane viewed along the C2-C3 sigma bond CH 3 CH 3 CH 3 CH 3 CH 3 H 3 C CH 3 H H H 3 C H H H H CH 3 H H CH 3 H H H H H H H H H H H H H H 3 C H H CH 3 H 0 60 120 180 240 300 E 0 60 120 180 240 300 360 C1-C2--C3-C4 dihedral angle, degrees clockwise
Conformational analysis of 2,3-dimethylhexane viewed along the C3-C4 sigma bond H CH 3 H H H 3 C H H H H H H H H H H H H CH 3 H CH 3 H 3 C H H 3 C H 180 120 60 0 300 240 E 0 60 120 180 240 300 360 C2-C3--C4-C5 dihedral angle, degrees clockwise
Conformations of cycloalkanes • Baeyer observed that the planar arrays of sp 3 - hybridized carbons which make up the constitutions of cylcoalkanes are generally distorted from the tetrahedral geometry: � CCC = 180(n-2) ° , n where n = the number of C in the cycloalkane parent ring. � CCC 60 ° 90 ° 108 ° 120 ° 129 ° • So, the relative thermodynamic stability of cycloalkanes ( � E / CH 2 ) should vary as a function of the Baeyer angle or ring strain .
Obviously, Baeyer’s predictions were not accurate! Why? • Cycloalkanes are not necessarily flat: they may be able to make conformational changes that can reduce ring strain. • The thermodynamic stability of a molecule is a function of angle strain + torsional strain + steric interactions • These factors are not independent: changing one can increase or decrease the others • Molecules will adjust their conformations so as to minimize strains and interactions. • Cyclohexane appears to be able to adopt a conformation with no angle strain . Can this conformation be determined?
Cyclohexane All-staggered, chair conformations:
H H H H H H H H H H H H H H H H H H H H H H H H H H H H H 2 H 2 H C H H C H H C H H C H H 2 H 2 H H H H
H H H H H H half-chair half-chair H H H H H H H H H H H H H H H H H H 10.8 6.4 H H H H H H H H H H H H 5.5 boat H H H H H H H H H H H H H H H H H H H H H H H H twist-boat twist-boat H H H H H H H H H H H H H H chair chair H H H H H H H H H H
Alkylcyclohexanes: gauche vs. anti conformations and 1,3-diaxial interactions H H H HH H H H H H H H H H H H H CH 2 H CH 2 H H H H H CH 2 CH 2 CH 3 CH 3 H H H with CH 3 axial, it is gauche to CH 2 , but with CH 3 equatorial, it is anti to CH 2 H H H H H H H H another way to look at this gauche interaction: 1,3-diaxial interactions between axial substituents on the same side of the ring
Hydrocarbons with more than 1 ring Spirocycles contain 1 C common to 2 rings � O spiro[4.5]decane β -vetivone
Hydrocarbons with more than 1 ring Polycycles contain � 2 C common to � 2 rings � they have “shared sides” OH O bicyclo[4.4.0] octane testosterone OH O
O bicyclo[3.2.1]octane camphor
Hydrocarbons with more than 1 ring Catenanes share no C � a [2]catenane a trefoil knot
Twistane, C 20 H 40 Olympiadane (1994) (a [5]catenane)
Recommend
More recommend