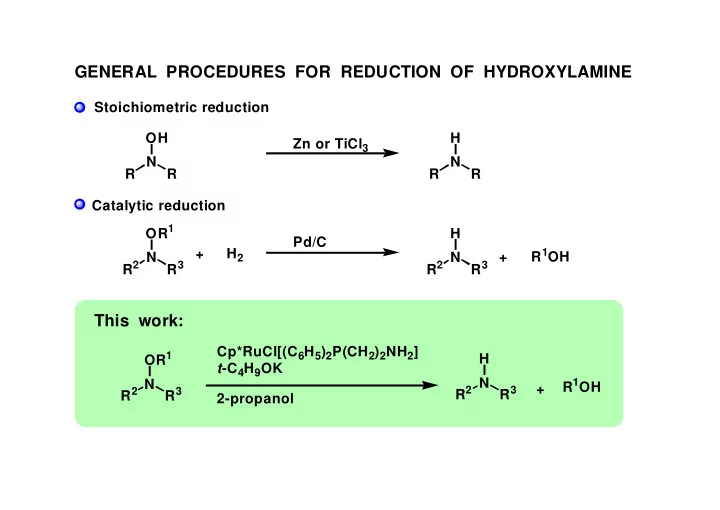

GENERAL PROCEDURES FOR REDUCTION OF HYDROXYLAMINE Stoichiometric reduction OH H Zn or TiCl 3 N N R R R R Catalytic reduction OR 1 H Pd/C + H 2 R 1 OH + R 2 N R 2 N R 3 R 3 This work: Cp*RuCl[(C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 ] OR 1 H t -C 4 H 9 OK R 2 N R 1 OH R 2 N + R 3 R 3 2-propanol
LIGAND EFFECT Cp*RuCl(L–L) OH H O t -C 4 H 9 OK Ph N Ph Ph N Ph Ph N Ph Ph N Ph + + 2-propanol 80 °C, 18 h S/C=100, Ru: t -C 4 H 9 OK=1:2, [hydroxylamine] = 0.5 M yield, % conv, % a amine a nitron a imine b L–L sel, % 81 77 <1 <1 >99 (C 6 H 5 ) 2 P NH 2 59 49 6 2 83 (C 6 H 5 ) 2 P NHCH 3 98 66 19 7 68 (C 6 H 5 ) 2 P N(CH 3 ) 2 >99 86 5 7 86 N NH 2 98 67 20 7 68 (CH 3 ) 2 N NH 2 >99 56 29 8 57 a Isolated yield. b Determined by 1 H NMR.
HYDROGEN SOURCE H 2 Cp*RuCl(L–L) OH O OH H t -C 4 H 9 OK + H 2 O + + Ph N Ph Ph N Ph 2-propanol 80 °C, 2 h S/C = 100, Ru: t -C 4 H 9 OK = 1:2, [hydroxylamine] = 0.5 M conv, % a yield, % b H 2 , atm L–L 0 42 41 (C 6 H 5 ) 2 P NH 2 5 18 16 0 75 43 (CH 3 ) 2 N NH 2 5 71 36 a Determined by NMR. b Isolated yield.
SOLVENT EFFECT Cp*RuCl[(C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 ] OH H t -C 4 H 9 OK Ph N Ph Ph N Ph + H 2 O solvent–2-propanol 80 °C, 18 h S/C = 100, Ru: t -C 4 H 9 OK = 1:2, [hydroxylamine] = 0.5 M conv, % a yield, % a solvent sel, % 2-propanol 81 77 >99 toluene 44 36 >99 CH 3 CN 32 10 31 Condition: Solvent 3 ml, 2-propanol 1 ml a Isolated yield.
EFFECT OF SUBSTITUENTS Cp*RuCl[(C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 ] OR 1 H t -C 4 H 9 OK R 2 N R 2 N R 1 OH + R 3 R 3 2-propanol 80 °C, 2 h S/C = 10, Ru: t -C 4 H 9 OK = 1:2, [hydroxylamine] = 0.1 M yield, % a hydroxylamine conv, % a amine alcohol OH Ph N >99 Ph NH 2 0 H OH H Ph N Ph >99 74 Ph N Ph NH 2 n.d. b >99 NH 3 85 Ph O Ph OH O Ph >99 Ph NH 2 30 88 Ph NH Ph OH O Ph no reaction Ph N Ph a Determined by 1 H NMR & 13 C NMR. b n.d. not determined.
REDOX DISPROPORTIONATION OF HYDROXYLAMINE Cp*RuCl[(C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 ] t -C 4 H 9 OK OH H O + Ph N Ph Ph N Ph Ph N Ph benzene-d6, 80 °C, 24 h 89% conv. ca 1 : 1 S/C = 100, Ru: t -C 4 H 9 OK = 1:2, [hydroxylamine] = 0.5 M REDUCTION OF NITRON WITH ALCOHOLS Cp*RuCl[(C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 ] t -C 4 H 9 OK H OH O + + Ph N Ph Ph N Ph Ph N Ph Ph N Ph 2-propanol, 80 °C, 18 h 95% conv. 73% a yield 18% a 4% b a isolated yield S/C = 100, Ru: t -C 4 H 9 OK = 1:2, [nitron] = 0.5 M b determined by NMR
REDUCTION OF N- OXIDE WITH ALCOHOLS CH 3 CH 3 O Cp*RuCl[(C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 ] t -C 4 H 9 OK N N + H 2 O 2-propanol, 80 °C, 18 h O O >99% yield determined by GC S/C = 100, Ru: t -C 4 H 9 OK = 1:2, [ N -oxide] = 0.5 M REDUCTION OF IMINE WITH ALCOHOLS Cp*RuCl[(C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 ] t -C 4 H 9 OK H N Ph N Ph CH 3 2-propanol, 30 °C, 24 h CH 3 30% yield determined by NMR S/C = 100, Ru: t -C 4 H 9 OK = 1:2, [imine] = 0.5 M
A POSSIBLE MECHANISM OH [Cp*Ru(PN)] R N R [Ru] [Cp*Ru(PN)]-H 2 [Cp*Ru(PN)] [Cp*Ru(PN)]-H 2 O + H 2 O N R R + N R R H N R R Ar [Cp*Ru(PN)]-H 2 Ar P + H Ru H 2 O δ - [Cp*Ru(PN)] N H δ + H polar substrates
SUMMARY Cp*RuCl[(C 6 H 5 ) 2 P(CH 2 ) 2 NH 2 ] OR 1 H t -C 4 H 9 OK R 1 OH R 2 N R 2 N + R 3 R 3 2-propanol Catalytic N–O single bond cleavage High chemoselectivity Reductive transformation of hydroxylamine leading to amine & alcohol
Recommend
More recommend