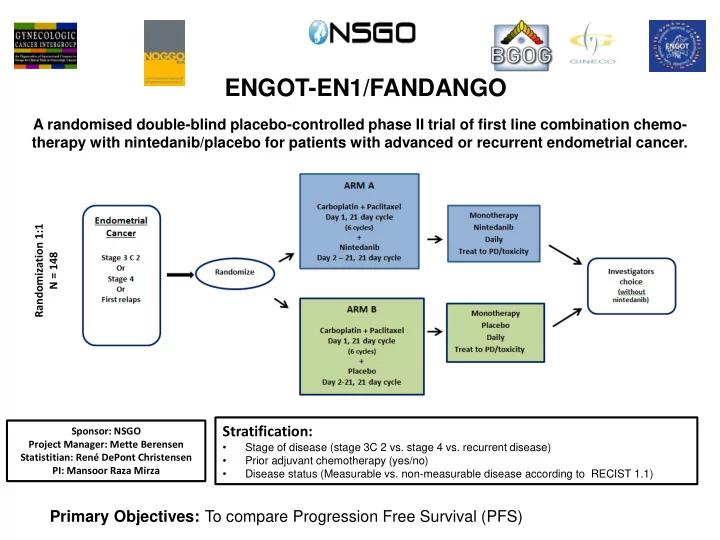

ENGOT-EN1/FANDANGO A randomised double-blind placebo-controlled phase II trial of first line combination chemo- therapy with nintedanib/placebo for patients with advanced or recurrent endometrial cancer. Randomization 1:1 N = 148 Stratification: Sponsor: NSGO Project Manager: Mette Berensen • Stage of disease (stage 3C 2 vs. stage 4 vs. recurrent disease) Statistitian: René DePont Christensen • Prior adjuvant chemotherapy (yes/no) PI: Mansoor Raza Mirza • Disease status (Measurable vs. non-measurable disease according to RECIST 1.1) Primary Objectives: To compare Progression Free Survival (PFS)
ENGOT-EN1/FANDANGO Inclusion criteria Histological confirmed endometrial cancer. • Stage 3C 2 • Stage 4 A & B • Relapsed after adjuvant therapy for stage 1-3 disease Prior therapy 2. Patients may have undergone primary surgery. 3. Patients may have received adjuvant chemotherapy for stage 1 – 3. 4. Patients may have received vaginal brachytherapy 5. Patients may have received external beam radiotherapy. Patients who are to be enrolled for stage 3C2 diseases are allowed to receive external beam radiotherapy prior to trial entry. 6. Patients may have received hormonal treatment Disease status 7. Patients must have measurable disease or non-measurable disease on CT scan according to RECIST 1.1 outside irradiated field. For stage 3C2 disease patients without measureable or non-measureable disease are accepted.
ENGOT-EN1/FANDANGO Randomized Patients 160 140 120 100 Number of patients 80 60 40 20 0 Nov 16 Dec 16 Jan 17 Feb 17 Mar 17 Apr 17 May 17 Jun 17 Jul 17 Aug 17 Sep 17 Okt 17 Nov 17 Dec 17 Jan 18 Feb 18 Mar 18 Apr 18 May 18 Expected Randomized patients Randomized patients 148 Patients in total
Recommend
More recommend