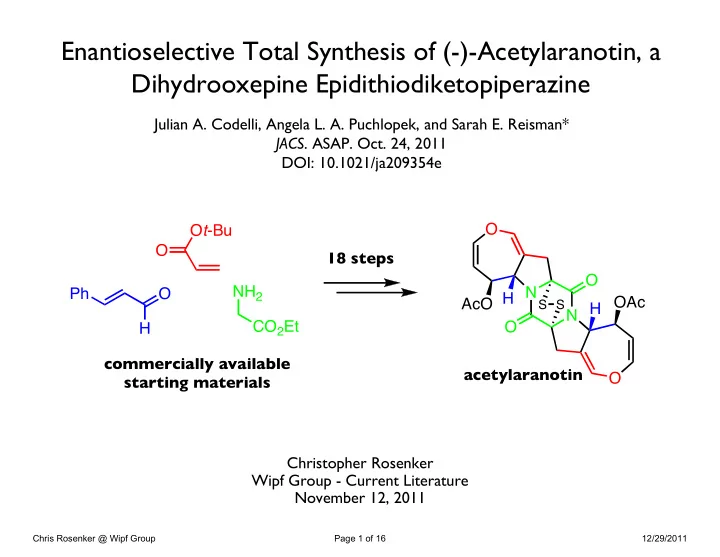

Enantioselective Total Synthesis of (-)-Acetylaranotin, a Dihydrooxepine Epidithiodiketopiperazine Julian A. Codelli, Angela L. A. Puchlopek, and Sarah E. Reisman* JACS . ASAP. Oct. 24, 2011 DOI: 10.1021/ja209354e O O t -Bu O 18 steps O NH 2 N Ph O AcO H H OAc S S N CO 2 Et O H commercially available acetylaranotin O starting materials Christopher Rosenker Wipf Group - Current Literature November 12, 2011 Chris Rosenker @ Wipf Group Page 1 of 16 12/29/2011
Epidithiodiketopiperazine natural products Epidithiokiketopiperazines (ETP) are a broad collection of fungal metabolites that contain at least 14 different core structures. •Biosynthetically arise by the joining of two amino acids which are further functionalized via oxidative pathways •Subset contains a 7-membered dihydrooxepine ring Biological activity of dihydrooxepine containing ETPs include inhibition of viral RNA polymerase and antiproliferative/apoptotic activity against human cancer cells. O O O O N H S S O O O Ph O N N N AcO H O O O H OAc O H S S S S N O H N H HO N S S O O OH O N HO O Ph O O OH OMe ( ! )-emethallicin A ( ! )-acetylaranotin MeO ( ! )-gliotoxin ( + )-MPC1001B Gardiner, D. M.; Waring, P.; Howlett, B. J. Microbiology 2005 , 151 , 1021. Codelli, J. A.; Puchlopek, A. L. A.; Reisman, S. E. J. Am. Chem. Soc. 2011 , DOI: 10.1021/ja209354e. Chris Rosenker @ Wipf Group Page 2 of 16 12/29/2011
Biosynthetic proposal of gliotoxin and aranotin type natural products The biosynthesis of gliotoxin and aranotin type natural products is hypothesized to occur via an epoxidized benzene ring. O O O [O] N HN HN OHH O gliotoxin type ring expansion O O O O O O [O] N HN HN H O OH aranotin type Neuss, N.; Boeck, L. D.; Brannon, D. R.; Cline, J. C.; DeLong, D. C.; Gorman, M.; Huckstep, L. L.; Lively, D. H.; Mabe, J.; Marsh, M. M.; Molloy, B. B.; Nagarajan, R.; Nelson, J. D.; Stark, W. M. Antimicrob. Agents Chemother. 1968 , 213. Gardiner, D. M.; Waring, P.; Howlett, B. J. Microbiology 2005 , 151 , 1021 . Chris Rosenker @ Wipf Group Page 3 of 16 12/29/2011
Synthetic Methodologies to from Oxepines Cadogan, Gosney & Co-workers : Cope rearrangement using a highly strained epoxycyclobutane. 10 -3 mmHg O HCO 3 H SO 2 O SO 2 O (39%) 580 °C (55%) White & Co-workers : Cope rearrangement of cis -divinyl epoxide. O O LiHMDS; O CCl 4 , 135 °C AcO 2 -70 °C OAc O OAc 12 h (94%) (70%) TMS TMS TMS Aitken, R. A.; Cadogan, J. I. G.; Gosney, I.; Hamill, B. J.; McLaughlin, L. M. J. Chem. Soc.; Chem. Comm. 1982 , 1164. Clark, D. L.; Chou, W.-N.; White, J. B. J. Org. Chem. 1990 , 55 , 3975. Chris Rosenker @ Wipf Group Page 4 of 16 12/29/2011
Synthetic Methodologies to from Oxepines Snapper & Leyhane : Thermal fragmentation of highly strained epoxides provides functionalized oxepines. H H H CO 2 Me CO 2 Me CO 2 Me CO 2 Me m -CPBA 200 °C, 2 h H H O H H BHT, PhH O O CH 2 Cl 2 (93%) H O O [0.01 M] O O 35% 36% O O CO 2 Me CO 2 Me H H H H H a CO 2 Me O O O O H H H H H H H H H CO 2 Me b CO 2 Me O O O O Leyhane, A. J.; Snapper, M. L. Org. Lett. 2006 , 8 , 5183. Chris Rosenker @ Wipf Group Page 5 of 16 12/29/2011
Synthetic Methodologies to from Oxepines Vargas & Co-workers : Isolated oxepine during structural elucidation of sesquiterpene hydroperoxide. OAc OH OAc O Ac OH Ac 2 O, pyridine O O O O O O (31%) Ph O H Ph O H Ph O H H Pyr: Kishi & Goodman : Criegee rearrangement of allylic hydroperoxides to form an Aranotin type precursor. HOO O O 1. ( p -NO 2 Bz) 2 O, DMAP p -NO 2 BzO (5 mol%), -45 to -20 °C 0.45 torr 2. BF 3 ·Et 2 O (47 mol%) 150 to 250 °C N N N TBDPSO H TBDPSO H H TBDPSO -40 to -20 °C, 6 h (70%) BzBr BzBr BzBr (45% over two steps) O p -NO 2 Ph O O N H TBDPSO BzBr Lu, T.; Vargas, D.; Fischer, N. H. Phytochemistry 1993 , 34 , 737. Goodman, R.; Kishi, Y. J. Am. Chem. Soc. 1998 , 120 , 9392. Goodman, R. M. Ph.D. Thesis, Harvard University, May 1998. Chris Rosenker @ Wipf Group Page 6 of 16 12/29/2011
Synthetic Methodologies to from Oxepines Fustero & Co-workers : Tandem RCM-olefin isomerization methodology. Ph O O Ph O O O Grubbs II Grubbs II toluene, reflux toluene, reflux (60%) 23% 46% Bräse & Co-workers : Enol-ether RCM on an unprotected allylic alcohol. OH [Ir(cod)Cl] 2 (10 mol%) Grubbs II, Grubbs-Hoveyda, O O H NaHCO 3 , vinyl acetate Schrock catalysts toluene, 100 °C CO 2 t -Bu CO 2 t -Bu N N TBSO TBSO H H (95%, E:Z;8:2) CO 2 t -Bu N Boc TBSO Boc H Boc O H 1. TBAF, THF (87%) CO 2 t -Bu 2. Grubbs II (20 mol%) N HO H toluene, 110 °C (quant.) Boc Fustero, S.; Sáchez-Rosell ó , M.; Jiménez, D.; Sanz-Cervera, J. F.; del Pozo, C.; Aceña, J. L. J. Org. Chem. 2006 , 71 , 2706. Gross, U.; Nieger, M.; Bräse, S. Chem.-Eur. J. 2010 , 16 , 11624. Chris Rosenker @ Wipf Group Page 7 of 16 12/29/2011
Synthetic Methodologies to from Oxepines Clive & Peng : Acid-induced cyclicization of pendant alcohol onto a vinylogous amide. NMe 2 SiMe 3 SiMe 3 SePh O O SePh HO (MeO) 2 CH(NMe 2 ) TFA, toluene S S HO O O THF (70% brsm) 50 °C (77%) N N H H MEMO MEMO N N O O O O SiMe 3 SiMe 3 N O O H S S O O O O N NaIO 4 , THF-H 2 O O S S PhSe O O OH N (39%) N MEMO H MEMO H N N O O O OMe MeO ( + )-MPC1001B Peng, J.; Clive, D. L. J. Org. Lett. 2007 , 9 , 2939. Peng, J.; Clive, D. L. J. J. Org. Chem. 2009 , 74 , 513. Chris Rosenker @ Wipf Group Page 8 of 16 12/29/2011
Synthetic Methodologies to from Oxepines McDonald & Co-workers W-cat cycloisomerization MeO H O H W(CO) 5 O HO OH 1. cat. W(CO) 6 , Et 3 N HO OH 1. THF, h ! , 55 °C AcO Et 3 N, THF, 60 °C AcO 2. Ac 2 O, DMAP O O O O 2. Ac 2 O, Et 3 N, DMAP O O O O 61-82% 82% Esteruelas, Saá & Co-workers Jia & Co-workers Os-cat cylcoisomerization Ru-cat cylcoisomerization H R 1 R 1 PPh 2 [CpOs(py) 3 ]PF 6 OH O N N R 2 R 4 pyridine, 90 °C R 2 Ru O R 4 N R 3 R 3 O O H 56-68% THF, 80 °C HO 91% Alcázar, E.; Pletcher, J. M.; McDonald, F. E. Org. Lett. 2004 , 6 , 3877. Koo, B.; McDonald, F. E. Org. Lett. 2007 , 9 , 1737. Liu, P. N.; Su, F. H.; Wen, T. B.; Sung, H. H. Y.; Williams, I. D.; Jia, G. Chem.-Eur. J. 2010 , 16 , 7889. Varela-Fernández, A.; García-Yebra, C.; Varela, J. A.; Esteruelas, M. A.; Saá, C. Angew. Chem. Int., Ed. 2010 , 49 , 4278. Chris Rosenker @ Wipf Group Page 9 of 16 12/29/2011
Reisman Group Retrosynthetic Analysis metal-catalyzed cycloisomerization O O O H O O N N AcO H HO H H OAc H OH S S CO 2 Et N N N O O TBSO H H Teoc O O ( ! )-acetylaranotin catalytic asymmetric (1,3)-dipolar cycloaddition O t -Bu O t -Bu H O H H H O O NH 2 Ph O CO 2 Et CO 2 Et N N H H H TBSO CO 2 Et Ph Teoc H commercially available starting materials (-)-acetylaranotin was isolated over 40 years ago and had not yet been synthesized Codelli, J. A.; Puchlopek, A. L. A.; Reisman, S. E. J. Am. Chem. Soc. 2011 , DOI: 10.1021/ja209354e. Chris Rosenker @ Wipf Group Page 10 of 16 12/29/2011
Catalytic asymmetric (1,3)-dipolar cycloaddition 1. CuI (10 mol %) t -BuO 2 C HO 2 C brucin-OL (10 mol %) DBU (10 mol %) (50%, 96% ee ) Ph CO 2 Et N 2. TFA, Et 3 SiH, CH 2 Cl 2 (77%) H Ph N CO 2 Et ·TFA >98% ee OMe OMe OMe O OMe O O OsO 4 , NMO OMe OMe N acetone, t -BuOH H N N H H H H H 2 O H O O O H H H H H H H N H N N O O Cu HO OH ( ! )-brucine O H O $3.34/g (Aldrich) Ph N 95% OEt brucin-OL t -BuO Codelli, J. A.; Puchlopek, A. L. A.; Reisman, S. E. J. Am. Chem. Soc. 2011 , DOI: 10.1021/ja209354e. Kim, H. Y.; Shih, H.-J.; Knabe, W. E.; Oh, K. Angew. Chem., Int. Ed.. 2009 , 48 , 7420 . Chris Rosenker @ Wipf Group Page 11 of 16 12/29/2011
Synthesis of Acetylaranotin O O H HO 2 C MgBr 1. Teoc-OSu, Et 3 N H HO O H 2 O/dioxane (83%) THF, ! 78 to 0 °C; HO Ph CO 2 Et CO 2 Et N N HO CO 2 Et 2. O 3 , CH 2 Cl 2 , ! 78 °C; H H N H then DMS (93%) Teoc ·TFA Teoc Teoc: TMS O O OH O 1. TBSOTf, 2,6-lutidine then PPh 3 , DIAD H NaBH 4 , EtOH CH 2 Cl 2 , 0 °C (85%) CH 2 Cl 2 , 0 °C (76%) O (86%) CO 2 Et 2. AcOH, THF, H 2 O CO 2 Et N N HO H H (3:1:1) (79%) Teoc Teoc OH O H DMP, pyr, CH 2 Cl 2 (93%) CO 2 Et CO 2 Et N N TBSO TBSO H H Teoc Teoc Codelli, J. A.; Puchlopek, A. L. A.; Reisman, S. E. J. Am. Chem. Soc. 2011 , DOI: 10.1021/ja209354e. Chris Rosenker @ Wipf Group Page 12 of 16 12/29/2011
Synthesis of Acetylaranotin: dihydrooxepine formation O H O Conditions Conditions [Rh(cod)Cl] 2 , [Rh(cod)(MeCN) 2 ]BF 4 , CpRuCl[(4-FC 6 H 4 ) 3 P] 2 (CO) 5 W=C(OMe)Me, AuCl, Pd(OAc) 2 , CuI, AgOTf CO 2 Et CO 2 Et N N w/ and w/o ligands and additives TBSO H TBSO H Teoc Teoc OH O [Rh(cod)Cl] 2 (5 mol %) Cl Cl NCS, CH 2 Cl 2 (4-FC 6 H 4 ) 3 P (60 mol %) pyrrolidine·TFA; CO 2 Et CO 2 Et DMF, 85 °C (88%) N NaBH 4 , EtOH (93%) N TBSO TBSO H H Teoc Teoc A O O LiCl, Li 2 CO 3 , DMF desilylation 1. TBAF, THF, 0 °C (84%) 100 °C (53%) A CO 2 Et CO 2 Et N N 2. LiCl, Li 2 CO 3 , DMF TBSO H TBSO H H 100 °C (65%) Teoc mixture of mono- & bis-desilylated products Codelli, J. A.; Puchlopek, A. L. A.; Reisman, S. E. J. Am. Chem. Soc. 2011 , DOI: 10.1021/ja209354e. Chris Rosenker @ Wipf Group Page 13 of 16 12/29/2011
Recommend
More recommend