Electroporation MIT 3.042 Project Ami Yamamoto Joy Yuan Jennifer - PowerPoint PPT Presentation
Electroporation MIT 3.042 Project Ami Yamamoto Joy Yuan Jennifer Liang John Tejada Paulo Jacobs From Last Time Goal: To design a more durable water disinfectant system that requires lower energy input. Method: Electroporation to lyse
Electroporation MIT 3.042 Project Ami Yamamoto Joy Yuan Jennifer Liang John Tejada Paulo Jacobs
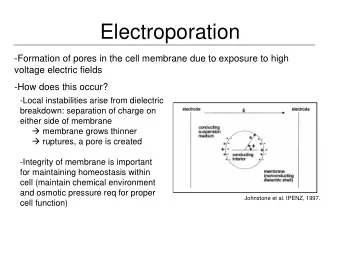
From Last Time… Goal: To design a more durable water disinfectant system that requires lower energy input. Method: Electroporation to lyse bacteria cells. Formation of pores in the cell membrane due to exposure to high voltage electric fields Proposed Design: 2 parallel electrode sheets + Power Supply Water Water & _ Pump
Electrode Material � Requirements � Minimal corrosion at the surface � Relatively inert with water � Operate under high voltage � Potential Candidates � Titanium Cathode : Ti � Low errosion rate Anode : � Commonly used for electroporation Anodized Ti � Can be anodized: corrosion-resistant � Stainless Steel � Lowest errosion rate
Pressure Limitations Derived from Couette flow: ∆ P = 3µLQ 2W δ 3 pressure difference vs. gap size Set electrode dimensions: 5 cm x 5 cm 16 14 Set flow rate Q: 12 pressure diff (atm) 1 liter/hour 10 8 achievable pressure differential To remain in a zone of 6 achievable pressure 4 differential, must have 2 gap size greater than 0 20 microns. 0 10 20 30 40 50 60 70 80 gap size (microns)
Pressure Limitations Derived from Couette flow: ∆ P = 3µLQ 2W δ 3 Set electrode dimensions: pressure difference vs. flow rate 5 cm x 5 cm 5 Set gap size 2 δ : Flow rate = 4 pressure diff (atm) 25 microns 1 liter/hour 3 To achieve a target flow 2 rate of at least 1 liter/hour, must have pressure 1 difference greater than 0 2 atm. 0.E+00 1.E-07 2.E-07 3.E-07 4.E-07 5.E-07 6.E-07 flow rate (m^3/s)
Voltage Limitations + Electric Field needed for Lysis: _ E = 1-5 × 10 5 V/m P = IV = ∆ E ∆ t ∆ E = V 2 ∆ t R V = Ed Verhes. Water Research, 2002.
Voltage Limitations Breakdown potential of H 2 O = 1.23V Gap size = 12.3 µm V = Ed Diameter of E. coli = 2-6 µm + _ Power Outlet = 50V Gap size = 500 µm = 0.5 mm
+ _ E Flow Orientation r
_ r Flow Orientation E +
Cylindrical Configuration
Final Shape Design
Recommend
More recommend
Explore More Topics
Stay informed with curated content and fresh updates.