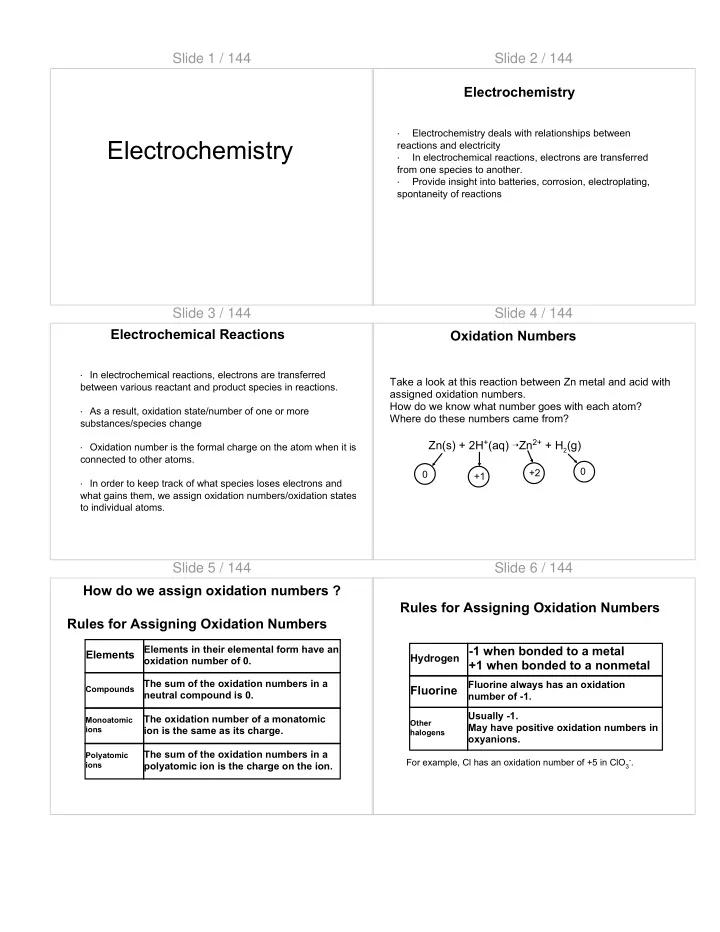

Slide 1 / 144 Slide 2 / 144 Electrochemistry · Electrochemistry deals with relationships between Electrochemistry reactions and electricity · In electrochemical reactions, electrons are transferred from one species to another. · Provide insight into batteries, corrosion, electroplating, spontaneity of reactions Slide 3 / 144 Slide 4 / 144 Electrochemical Reactions Oxidation Numbers · In electrochemical reactions, electrons are transferred Take a look at this reaction between Zn metal and acid with between various reactant and product species in reactions. assigned oxidation numbers. How do we know what number goes with each atom? · As a result, oxidation state/number of one or more Where do these numbers came from? substances/species change Zn(s) + 2H + (aq) ➝ Zn 2+ + H 2 (g) · Oxidation number is the formal charge on the atom when it is connected to other atoms. 0 +2 0 +1 · In order to keep track of what species loses electrons and what gains them, we assign oxidation numbers/oxidation states to individual atoms. Slide 5 / 144 Slide 6 / 144 How do we assign oxidation numbers ? Rules for Assigning Oxidation Numbers Rules for Assigning Oxidation Numbers Elements in their elemental form have an Hydrogen -1 when bonded to a metal Elements oxidation number of 0. +1 when bonded to a nonmetal Compounds The sum of the oxidation numbers in a Fluorine always has an oxidation Fluorine neutral compound is 0. number of -1. Usually -1. The oxidation number of a monatomic Monoatomic Other May have positive oxidation numbers in ions ion is the same as its charge. halogens oxyanions. The sum of the oxidation numbers in a Polyatomic For example, Cl has an oxidation number of +5 in ClO 3 - . ions polyatomic ion is the charge on the ion.
Slide 7 / 144 Slide 8 / 144 1 What is the oxidation number of each oxygen Rules for Assigning Oxidation Numbers atom in the compound MnO 2 ? A -2 Nonmetals tend to have negative B -1 Nonmetals oxidation numbers although some are positive in certain compounds or ions. C 0 Oxygen has a oxidation number of -2, D +1 Oxygen except in the peroxide ion, when its E +2 oxidation number is -1. Slide 9 / 144 Slide 10 / 144 3 What is the oxidation number of oxygen atom in 2 What is the oxidation number of the manganese MnO 4 1- , the permanganate ion? atom in the compound MnO 2 ? +3 A -2 A +2 B -1 B C 0 C +1 D D +4 +2 E +7 E +4 Slide 11 / 144 Slide 12 / 144 4 What is the oxidation number of the manganese 5 What is the oxidation number of sulfur in HSO 4 1- , atom in MnO 4 1- , the permanganate ion? the hydrogen sulfate ion? A +1 A -2 B +2 +1 B C +5 +2 C D +4 +4 D +7 E E +6
Slide 13 / 144 Slide 14 / 144 Oxidation and Reduction Oxidation and Reduction Zn(s) + 2H + (aq) ➝ Zn 2+ + H 2 (g) Zn(s) + 2H + (aq) ➝ Zn 2+ + H 2 (g) 0 +2 0 +1 +2 0 0 +1 Oxidation-loss of electrons A species is oxidized when it loses electrons. Here, zinc loses two electrons to go from neutral Zn metal to Reduction- gaining of electrons the Zn 2+ ion. A species is reduced when it gains electrons. Zn is also a reducing agent- provides electrons (reductant) Reducing agent loses electrons. Here, each of the H + gains an electron, and they combine to form H 2 . LEO H is an oxidizing agent- accepts electrons (oxidant) An oxidizing agent gains electrons. OIL The lion says RIG GER Slide 15 / 144 Slide 16 / 144 Oxidation and Reduction Oxidation and Reduction Redox Reactions Zn(s) + 2H + (aq) ➝ Zn 2+ + H 2 (g) 0 +2 0 +1 Zn(s) + 2H + (aq) ➝ Zn 2+ + H 2 (g) 0 +2 · What is reduced is the oxidizing agent. 0 +1 · H + oxidizes Zn by taking electrons from it. · What is oxidized is the reducing agent. An electrochemical reaction in which oxidation and · Zn reduces H + by giving it electrons. reduction occurs is known as a REDOX reaction Slide 17 / 144 Slide 18 / 144 7 Which substance is oxidized in the following 6 Which of the following is/are an oxidation- reduction (redox) reactions? reaction? (First, assign oxidation numbers.) (a) K 2 CrO 4 + BaCl 2 ➝ KCl + BaCrO 4 Cu + S ➝ CuS (b) Pb 2+ + 2 Br 1- ➝ PbBr 2 (c) Cu + S ➝ CuS A Cu B S a only A C Cu and S B b only D CuS C c only E This is not a redox reaction. a and c D b and c E
Slide 19 / 144 Slide 20 / 144 8 Which substance is the reducing agent below? 9 Which substance is oxidized in the following reaction? (First, assign oxidation numbers.) Cu + S ➝ CuS Ca + Fe 3+ ➝ Ca 2+ + Fe A Cu A Ca Fe 3+ B S B Ca 2+ C Cu and S C D CuS D Fe E This is not a redox reaction. E This is not a redox reaction. Slide 21 / 144 Slide 22 / 144 10 Which substance is the oxidizing agent below? 11 Which substance is reduced in the following reaction? (First, assign oxidation numbers.) Ca + Fe 3+ ➝ Ca 2+ + Fe 3 K + Al(NO 3 ) 3 ➝ Al + 3 KNO 3 A Ca A K Fe 3+ B B Al Ca 2+ C N C D Fe D O E This is not a redox reaction. E This is not a redox reaction. Slide 23 / 144 Slide 24 / 144 12 Which substance is the reducing agent? Redox Practice 1 3 K + Al(NO 3 ) 3 ➝ Al + 3 KNO 3 H 2 S (g) + Cl 2 (g) --> 2HCl (g) + S (s) a) Assign oxidation numbers to each element above. A K b) Which element is oxidized? B Al(NO 3 ) 3 c) Which element is reduced? C KNO 3 d) Name the reducing agent. D This is not a redox reaction. e) Name the oxidizing agent.
Slide 25 / 144 Slide 26 / 144 Redox Practice 2 13 Which element is oxidized in the reaction below? 2- ➝ Fe 3+ + Cr 3+ + H 2 O Fe 2+ + H + + Cr 2 O 7 SnCl 2 (aq) + 2HgCl 2 (aq) --> SnCl 4 (aq) + Hg 2 Cl 2 (s) a) Assign oxidation numbers to each element above. b) Which element is oxidized? c) Which element is reduced? d) Name the reducing agent. e) Name the oxidizing agent. Slide 27 / 144 Slide 28 / 144 15 SnCl 2 (aq) + 2HgCl 2 (aq) --> SnCl 4 (aq) + Hg 2 Cl 2 (s) 14 H 2 S (g) + Cl 2 (g) --> 2HCl (g) + S (s) Which is oxidized? Which is oxidized? Which is reduced? Which is reduced? Slide 29 / 144 Slide 30 / 144 Redox reactions in aqueous solutions Redox reactions in aqueous solutions This reaction involves two parts as represented below. A large number of redox reactions occur in aqueous solutions. 2I- (aq) + H 2 O 2 (aq) + 2e- ⇒ I 2 + 2OH- (aq) + 2e- Unlike acid base nutralization and precipitation 1. Oxidation half reaction reactions,most of the reaction proceed slowly. 2. Reduction half reaction. Each redox reaction is the sum of two half reactions: 2I - (aq) ⇒ I 2 + 2e - oxidation H 2 O 2 (aq) + 2e - ⇒ 2OH - (aq) reduction Consider the reaction of iodide ions and hydrogen peroxide. Add the two half reactions to get the overall reaction. 2I- (aq) + H 2 O 2 (aq) + 2e- ⇒ I2 + 2OH- (aq) + 2e- 2I- (aq) + H 2 O 2 (aq) + 2e- ⇒ I 2 + 2OH- (aq) + 2e- How do we balance a redox reaction?
Slide 31 / 144 Slide 32 / 144 Balancing Redox reactions Half-reaction method (oxidation # method) Let us consider the simple replacement reaction of Mg Half-reaction method (oxidation # method) with AgCl Mg + AgCl ➝ Ag + MgCl 2 · Assign oxidation numbers to determine what is oxidized and what is reduced. -1 0 -1 · Identify the oxidation and reduction process. +1 0 +2 Oxidation: Mg --> Mg 2+ + 2 e - --------(1) · Write down the individual oxidation and reduction equations. Reduction: Ag + + 1 e - --> Ag ---------(2) · Balance these half reactions Since all the atoms are balanced, we need to balance only electrons. Multiply equation (2) x 2 · Combine them to attain the balanced equation for the overall reaction. Oxidation: Mg --> Mg 2+ + 2 e- --------(1) This method can be used in general to balance any redox reaction unless any specific condition such as Reduction: 2Ag + + 2 e- --> 2Ag ---------(3) acidic or basic is mentioned Slide 33 / 144 Slide 34 / 144 Half-reaction method (oxidation # method) Redox reactions -balancing Adding the half-reactions (1) and (3) yields the following: Practice Fe 3 O 4 +C --> Fe + CO Oxidation: Mg --> MgCl 2 + 2e - Reduction: 2AgCl + 2e - --> 2Ag Overall: Mg + 2AgCl + 2e - --> MgCl 2 + 2e - + 2Ag and we cancel out electrons from both sides: Overall: Mg + 2AgCl + 2e - --> MgCl 2 + 2e - + 2Ag Net equation: Mg + 2AgCl --> MgCl 2 + 2Ag Since the original equation is given with chlorine you would keep it here in the final balanced equation too. Fe3O4 + 4C --> 3Fe + 4CO Slide 35 / 144 Slide 36 / 144 The Half-Reaction Method Redox reactions -balancing In acidic medium: Practice: SnO 2 + C --> Sn + CO Other atoms This diagram shows the steps involved in balancing half-reactions. · Write down the individual half reaction. O · First balance atoms other than H and O. · Balance oxygen atoms by adding H 2 O. · Balance hydrogen atoms by adding H + . H · Balance charge by adding electrons. · Multiply the half-reactions by integers so e- that the electrons gained and lost are the same. SnO 2 + 2C --> Sn + 2CO
Recommend
More recommend