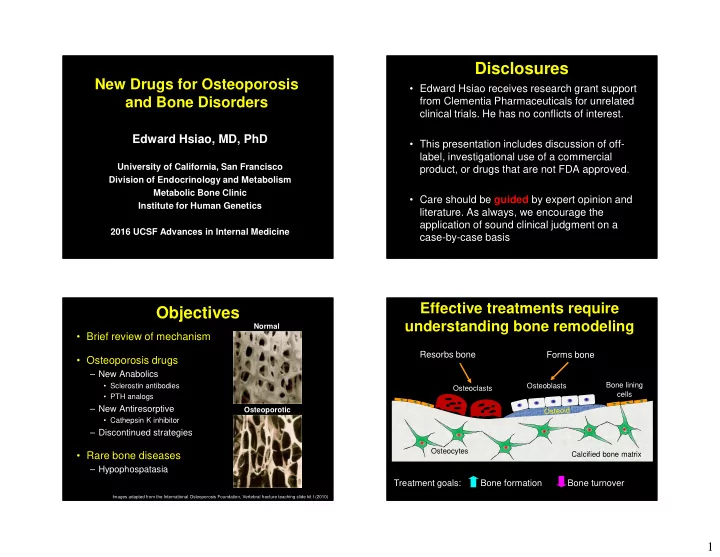

Disclosures New Drugs for Osteoporosis • Edward Hsiao receives research grant support and Bone Disorders from Clementia Pharmaceuticals for unrelated clinical trials. He has no conflicts of interest. Edward Hsiao, MD, PhD • This presentation includes discussion of off- label, investigational use of a commercial University of California, San Francisco product, or drugs that are not FDA approved. Division of Endocrinology and Metabolism Metabolic Bone Clinic • Care should be guided by expert opinion and Institute for Human Genetics literature. As always, we encourage the application of sound clinical judgment on a 2016 UCSF Advances in Internal Medicine case-by-case basis Effective treatments require Objectives understanding bone remodeling Normal • Brief review of mechanism Resorbs bone Forms bone • Osteoporosis drugs – New Anabolics • Sclerostin antibodies Bone lining Osteoblasts Osteoclasts cells • PTH analogs – New Antiresorptive Osteoporotic • Cathepsin K inhibitor – Discontinued strategies Osteocytes • Rare bone diseases Calcified bone matrix – Hypophospatasia Treatment goals: Bone formation Bone turnover Images adapted from the International Osteoporosis Foundation, Vertebral fracture teaching slide kit I (2010) 1
Current Treatments for Recent Changes in Drugs Osteoporosis for Osteoporosis Increase Bone Formation Decrease Bone Turnover • Parathyroid hormone • Hormone Therapy (HT) • Anabolics • Antiresorptives (rPTH, Teriparatide) • SERM/Raloxifene (Evista) – Anti-sclerostin – Cathepsin K inhibitor • Calcitonin (Miacalcin) antibodies • Ondanacatib • Bisphosphonates • Romosozumab • Blomosozunab – New considerations – Alendronate (Fosamax) for denosumab – Risedronate (Actonel) – Ibandronate (Boniva) – PTHrP analog – Restrictions on – Zoledronate (Reclast/Aclasta) • Abaloparatide strontium ranelate (in • (Strontium ranelate) EU market) • RANKL inhibitors – Vibration Therapy – Denosumab (Prolia) Sclerostin is a Key Mediator Recent Changes in Drugs of Bone Formation for Osteoporosis Sclerosteosis X Wnt Sclerostin X • Anabolics • Antiresorptives – Anti-sclerostin – Cathepsin K inhibitor antibodies LRP5/6 • Ondanacatib Frizzled • Romosozumab • Blomosozunab – New considerations for denosumab Normal – PTHrP analog – Restrictions on β -Catenin • Abaloparatide strontium ranelate (in EU market) Activation of bone – Vibration Therapy Formation pathways Brunkow et al. Am J Hum Gen 2001 2
Anti-Sclerostin Antibody #1: Romosozumab: Phase I Romosozumab • Additional Phase I study • Romosozumab (Amgen/UCB: AMG785, CDP7851) – Multiple doses – Humanized monoclonal antibody – 32 postmenopausal women with low bone mass • Phase 1 study: – Single dose of AMG785, 72 men and women; • 6 doses, 1-2 mg/kg every 2 weeks, or – Peak serum concentration achieved after 1 week sq • 3 doses of 2-34 mg/kg every 4 weeks, or – Half life of 11-18 days placebo – Dose ranging was done from 0.1-10 mg/kg – 16 healthy men with low bone mass. – 10mg/kg (maximum dose tested) • 1 mg/kg every 2 weeks, or • 120-184% increase in P1NP, BSAP, Osteocalcin • 54% decrease in CTX • 3 mg/kg every 4 weeks, or placebo • Largest BMD effect at day 85 • +5.3% lumbar spine BMD • + 2.8% in total hip BMD Padhi, et al. JBMR 2011 Padhi, et al. J Clin Pharm 2014 Romosuozumab Romosozumab: Phase II • 419 postmenopausal women – T score between -2.0 and -3.5 in spine, total hip or femoral neck. – Monthly sq (70, 140, or 210 mg) or every 3 months (140 or 210 mg), for 12 months – Open label comparison to • 12 weeks of drug, 12 weeks of followup • Alendronate 70 mg weekly • Teriparatide 20 ug/day • Appropriate changes in serum markers observed. • No major effect on hip BMD • Persistence of effect after dosing stopped Padhi, et al. J Clin Pharm 2014 3
Romosozumab Gives Romosozumab: Phase III Higher Increase in BMD • Recent announcements indicate that it meets goals, but full publications pending • STRUCTURE trial* (Sept 2015 press release) – 436 postmenopausal women previously treated with bisphosphonates – Romosozumab vs teriparatide – Met goals for total hip BMD • FRAME trial** (Feb. 2016 press release) – 7180 patients, 210 mg sq/month – Reduced incidence of new vertebral fractures at 12 and 24 mo • Every 3 months – same as 70 mg/mo dose – Reduced incidence of clinical fractures at 12 mo – Unclear benefit for non-clinical fractures at 12 and 24 mo. – Approx 5% increase in BMD at spine – Bone formation markers return to normal after 6-12 mo *STudy evaluating effect of RomosozUmab Compared with Teriparatide in postmenopaUsal women with osteoporosis at high risk for fracture pReviously treated with bisphosphonatE therapy ** FRActure study in postmenopausal woMen with ostEoporosis Anti-sclerostin Antibody #2: Romosozumab Side Effects Blosozumab (Reported in Phase I-III) • Injection site reactions • Humanized monoclonal antibody • No clear increase in serious adverse events – Eli Lilly (LY2541546) over alendronate, teriparatide • 20% develop binding antibodies, with 3% • Phase I trials showing in vitro blocking ability, but subjects – Single and multiple dose regimens tolerated up to still showed biologic response 750 mg every 2 weeks for 8 wks – 3.4-7.7% increase in lumbar BMD at Day 85 • Awaiting results of Phase III for full profile McColm, et al. JBMR 2014 4
Blosozumab: Phase II Blosozumab: Phase II Durability of treatment • 120 postmenopausal women, T score • Followup study for 1 year post treatment between -2.0 and -3.5 – 88 of 120 women previously studied Lumbar Spine Total Hip – Suggests antiresorptive will be needed Recker, et al. JBMR 2015 Recknor, et al. JBMR 2015 Osteoblast Activation by PTHrP: Recent Changes in Drugs Parathyroid Hormone Related Protein for Osteoporosis PTH/PTHrP Receptor • Anabolics • Antiresorptives PTH PTHrP – Anti-sclerostin – Cathepsin K inhibitor Bone lining Osteoblasts antibodies • Ondanacatib cells • Romosozumab • Blomosozunab – New considerations for denosumab – PTHrP analog – Restrictions on G s GPCR bone • Abaloparatide strontium ranelate (in anabolic response Calcified bone matrix EU market) – Vibration Therapy Treatment goals: Bone formation Bone turnover 5
Abaloparatide: A PTHrP analog Abaloparatide: Phase III • Synthetic peptide analog of human • ACTIVE fracture prevention trial PTHrP – 2463 postmenopausal women • Phase II: – 18 mo daily 80 ug abaloparatide vs placebo vs 20 ug teriparatide. – 24 weeks of daily sq injections – 89% decrease in new fracture rate vs. placebo • Postmenopausal women – Teriparatide showed an 80% decrease • No significant differences in wrist fractures • 20, 40, or 80 ug vs 20 ug of teriparatide • Increased BMD in spine and hip at 6, 12, and 18 months – Lumbar spine BMD increased 2.8-6.7%, vs – Major complications: hypercalcemia, and injection site 5.5% in teriparatide and 0.8% in placebo reactions. – Femoral neck increased 1.4-2.6%, vs. • Extension trial in progress 0.5% in teriparatide and 0.4% in placebo Leder, et al. JCEM 2015 2015 Endocrine Society poster: LB-OR01-3 Cathepsin K: Functions in Recent Changes in Drugs Osteoclast Resorption Pits for Osteoporosis Resorbs bone • Anabolics • Antiresorptives – Anti-sclerostin – Cathepsin K inhibitor Osteoclasts Cathepsin K antibodies • Ondanacatib - secreted by osteoclasts • Romosozumab - cleaves helical collagen • Blomosozunab – New considerations - induces bone resorption for denosumab – PTHrP analog – Restrictions on • Abaloparatide strontium ranelate (in Osteocytes EU market) – Vibration Therapy Treatment goals: Bone formation Bone turnover 6
Ondanacatib: Phase II Ondanacatib: Anti-Cathepsin K • Related to two other Cathepsin K inhibitors – Relacatib: nonselective inhibitor of K, L, V, and S • No clinical information • Kumar, et al. Bone 2007 (monkey model) – Balicatib: showed BMD increases, but had cutaneous adverse events. • Adami, et al. JBMR 2006 • 399 postmenopausal women – Ondanacatib: selective for Cathepsin K and orally – Generally well tolerated bioavailable. • Bone, et al. JBMR 2009 – No significant major side effects reported Denosumab Recent Changes in Drugs • Human monoclonal antibody that inhibits RANKL (required for for Osteoporosis osteoclast function and survival) • Given 60 mg sq every 6 months over 3 years reduces fracture risk (FREEDOM) and Freedom extension • Anabolics • Antiresorptives Vertebral Non- Hip N – Anti-sclerostin – Cathepsin K inhibitor vertebral (Ref #) antibodies • Ondanacatib Alendronate 0.55 0.84 0.60 12,068 • Romosozumab (10 mg qd) (0.43-0.69) (0.74-0.94) (0.40-0.92) (2) • Blomosozunab – New considerations Zoledronate 0.30 0.75 0.59 7,765 for denosumab (5 mg/yr iv) (0.24-0.38) (0.64-0.87) (0.42-0.83) (1) – PTHrP analog – Restrictions on Denosumab 0.32* 0.80* 0.60 7868 • Abaloparatide strontium ranelate (in (60 mg/6 mo sq) (0.26-0.41) (0.67-0.95) (0.37-0.97) (3) EU market) Relative risk of drug vs. placebo – Vibration Therapy * Hazard ratios (secondary endpoints of study) 1. Black, et al. NEJM 2007 (HORIZON) 2. Wells, et al. Cochrane DB or Syst. Rev. 2008 (CD001155 Alendronate) 3. Cummings, et al. NEJM 2009 (FREEDOM) 7
Recommend
More recommend