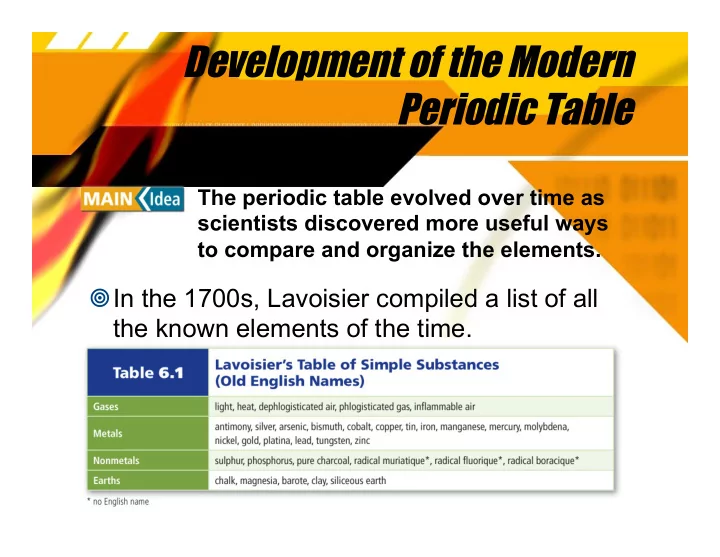

Development of the Modern Periodic Table The periodic table evolved over time as scientists discovered more useful ways to compare and organize the elements. ¥ In the 1700s, Lavoisier compiled a list of all the known elements of the time.
Development of the Modern Periodic Table ¥ The 1800s brought large amounts of information and scientists needed a way to organize knowledge about elements. ¥ John Newlands proposed an arrangement where elements were ordered by increasing atomic mass. ¥ Newlands noticed when the elements were arranged by increasing atomic mass, their properties repeated every eighth element. ¥ Meyer and Mendeleev both demonstrated a connection between atomic mass and elemental properties. ¥ Mendeleev published the first periodic table ¥ He left blank spaces for elements that had yet to be discovered
Development of the Modern Periodic Table ¥ Moseley rearranged the table by increasing atomic number, and resulted in a clear periodic pattern. ¥ Periodic repetition of chemical and physical properties of the elements when they are arranged by increasing atomic number is called periodic law .
Development of the Modern Periodic Table ¥ The modern periodic table contains boxes which contain the element's name, symbol, atomic number, and atomic mass.
Development of the Modern Periodic Table ¥ Columns of elements are called groups . ¥ Rows of elements are called periods . ¥ Elements in groups 1-8 possess a wide variety of chemical and physical properties and are called the representative elements . ¥ Elements in groups 3b-12b are known as the transition metals .
Development of the Modern Periodic Table ¥ Elements are classified as metals, non-metals, and metalloids. ¥ Metals are elements that are generally shiny when smooth and clean, solid at room temperature, and good conductors of heat and electricity. ¥ Alkali metals are all the elements in group 1 except hydrogen, and are very reactive. ¥ Alkaline earth metals are in group 2, and are also highly reactive.
Development of the Modern Periodic Table ¥ Non-metals are elements that are generally gases or brittle, dull-looking solids, and poor conductors of heat and electricity. ¥ Group 7 is composed of highly reactive elements called halogens . ¥ Group 8 gases are extremely unreactive and commonly called noble gases .
Development of the Modern Periodic Table ¥ Metalloids have physical and chemical properties of both metals and non-metals, such as silicon and germanium.
Bell Ringer Question ¥ Identify the group number, period number, and family name of the following elements: ¥ Calcium ¥ Fluorine
Classification of the Elements Elements are organized into different blocks in the periodic table according to their electron configurations. ¥ All group 1 elements have one valence electron.
Classification of the Elements ¥ The trend continues among each of the other main group elements (group 2 elements have 2 valence electrons, and group 3 elements have 3 valence electrons!)
Periodic Trends Trends among elements in the periodic table include their size and their ability to lose or attract electrons ¥ Trend 1 - Atomic Radius is a measure of the size of an atom
Periodic Trends ¤ Atomic Radius – The distance between the nucleus of two like atoms. Basically, the atomic radius is a measure of the size of an atom. ¤ Atomic Radius INCREASES down a group – new energy levels are being added which contributes to larger size. Each energy level further away from nucleus
Periodic Trends ¥ Atomic Radius DECREASES across a period. Why???? ¥ This is puzzling because protons, neutrons, and electrons are all INCREASING across a period, one would expect the atom would be larger.
Periodic Trends ¥ Electrons are filling an energy level as you move to the right across a period. Meanwhile, the nucleus is increasing in its positive charge. The electrons become MORE ATTRACTED to the nucleus…they squeeze in and make a more compact atom. ¥ Class example – What is your natural behavior toward people you are more attracted to? You MOVE CLOSER! To those who are less attractive? You MOVE FURTHER AWAY! ¥ Electrons are people too!!!
Periodic Trends
Periodic Trends ¥ Ionic Radius ¥ Ions that have LOST electrons to become POSITIVE get SMALLER (nucleus is more attractive to each electron) ¥ Ions that have GAINED electrons to become NEGATIVE get LARGER (nucleus is less attractive to each electron)
Periodic Trends
Periodic Trends ¥ Ionization Energy – Energy required to remove an electron from an atom. ¥ The key to understanding ionization energy to determine the “ happiness ” of the electron. The HAPPIER the electron is in its current location, the MORE ENERGY REQUIRED to remove it (the higher the ionization energy).
Periodic Trends ¥ Ionization energy INCREASES across a period, and DECREASES down a group. ¥ Elements at the left of the periodic table have very “ unhappy ” electrons. It is easy to remove one. Elements at the right of the periodic table have electrons that are much harder to remove, because they are “ happy ” where they are.
Periodic Trends ¥ Happiness is mostly determined by whether removing electrons helps get 8 valence electrons!!! ¥ Remember…Alkali Metals have ONE OUTER ELECTRON! If they lose it, the outer energy level goes away! This leaves 8 electrons in its new outer level!
Periodic Trends ¥ Class Example – Dictionary read with your dad. Are you happy or unhappy? How much energy does it take to remove you? Front row at favorite concert with your dad. Are you happy or unhappy? How much energy does it take to remove you? ¥ Electrons are people too!!!
Periodic Trends ¥ Elements at the bottom of the periodic table have electrons that are more “ unhappy ” than the elements at the top of the periodic table. ¥ Elements at the bottom of the periodic table are larger (greater atomic radius, remember the trend!) because they have more energy levels. Those electrons in the outer energy levels are attracted to the nucleus. BUT…all the other electrons are in their way! This is why they are unhappy! ¥ In chemistry we call this the SHIELDING EFFECT
Periodic Trends ¥ Class Example – Concert in the front row… how much energy does it take to remove you? Concert in the last row…how much energy does it take to remove you? ¥ Electrons are people too!!!
Periodic Trends ¥ The octet rule states that atoms tend to gain, lose or share electrons in order to acquire a full set of eight valence electrons. ¥ The octet rule is useful for predicting what types of ions an element is likely to form.
Periodic Trends ¥ The electronegativity of an element indicates its relative ability to attract electrons in a chemical bond. ¥ Electronegativity decreases down a group and increases left to right across a period. ¥ This is the same trend as ionization energy! ¥ Atoms with electrons that are happy (hard to lose) are more likely to invite additional electrons (easy to gain) to join the fun
Periodic Trends
Recommend
More recommend