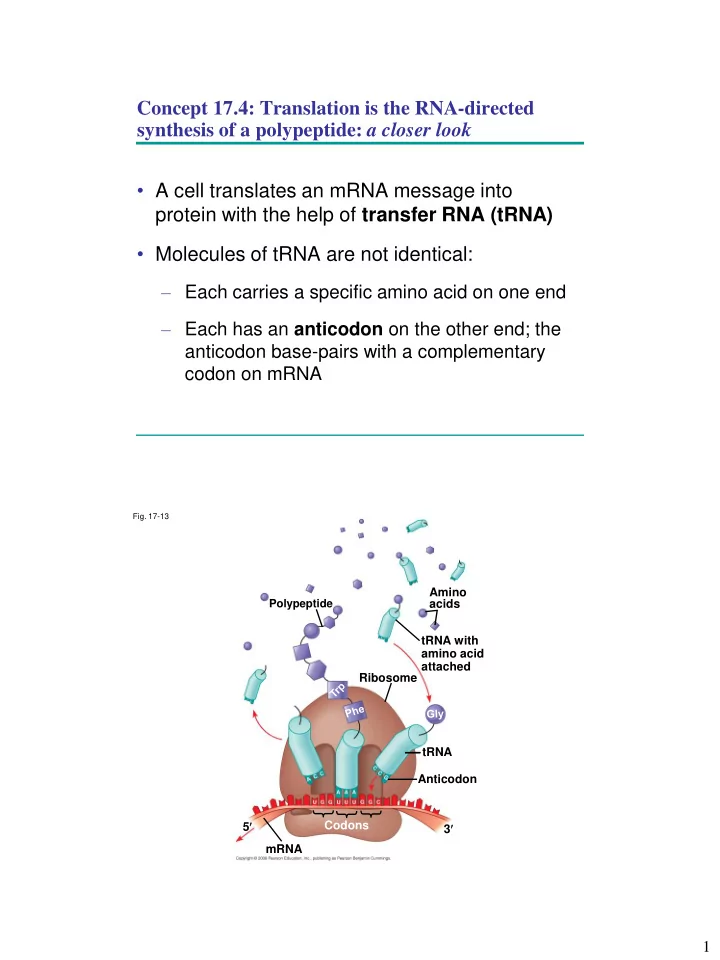

Concept 17.4: Translation is the RNA-directed synthesis of a polypeptide: a closer look • A cell translates an mRNA message into protein with the help of transfer RNA (tRNA) • Molecules of tRNA are not identical: – Each carries a specific amino acid on one end – Each has an anticodon on the other end; the anticodon base-pairs with a complementary codon on mRNA Fig. 17-13 Amino Polypeptide acids tRNA with amino acid attached Ribosome tRNA Anticodon 5 Codons 3 mRNA 1
The Structure and Function of Transfer RNA • A tRNA molecule consists of a single RNA A strand that is only about 80 nucleotides long C C • Flattened into one plane to reveal its base pairing, a tRNA molecule looks like a cloverleaf • Because of hydrogen bonds, tRNA actually twists and folds into a three-dimensional molecule • tRNA is roughly L-shaped Fig. 17-14 3 Amino acid attachment site 5 Hydrogen bonds Anticodon (a) Two-dimensional structure Amino acid 5 attachment site 3 Hydrogen bonds 3 5 Anticodon Anticodon (c) Symbol used (b) Three-dimensional structure in this book 2
• Accurate translation requires two steps: – First: a correct match between a tRNA and an amino acid, done by the enzyme aminoacyl- tRNA synthetase – Second: a correct match between the tRNA anticodon and an mRNA codon • Flexible pairing at the third base of a codon is called wobble and allows some tRNAs to bind to more than one codon Fig. 17-15-1 Aminoacyl-tRNA Amino acid synthetase (enzyme) P P P Adenosine ATP 3
Fig. 17-15-2 Aminoacyl-tRNA Amino acid synthetase (enzyme) P P P Adenosine ATP P Adenosine P P i P i P i Fig. 17-15-3 Aminoacyl-tRNA Amino acid synthetase (enzyme) P P P Adenosine ATP P Adenosine tRNA P P i Aminoacyl-tRNA P i P i synthetase tRNA P Adenosine AMP Computer model 4
Fig. 17-15-4 Aminoacyl-tRNA Amino acid synthetase (enzyme) P P P Adenosine ATP P Adenosine tRNA P P i Aminoacyl-tRNA P i P i synthetase tRNA P Adenosine AMP Computer model Aminoacyl-tRNA (“charged tRNA”) Ribosomes • Ribosomes facilitate specific coupling of tRNA anticodons with mRNA codons in protein synthesis • The two ribosomal subunits (large and small) are made of proteins and ribosomal RNA (rRNA) 5
Fig. 17-16 Growing polypeptide Exit tunnel tRNA molecules Large subunit E P A Small subunit 5 3 mRNA (a) Computer model of functioning ribosome P site (Peptidyl-tRNA binding site) A site (Aminoacyl- tRNA binding site) E site (Exit site) E P A Large subunit mRNA Small binding site subunit (b) Schematic model showing binding sites Growing polypeptide Amino end Next amino acid to be added to polypeptide chain E tRNA mRNA 3 5 Codons (c) Schematic model with mRNA and tRNA Fig. 17-16a Growing polypeptide Exit tunnel tRNA molecules Large subunit E P A Small subunit 5 3 mRNA (a) Computer model of functioning ribosome 6
Fig. 17-16b P site (Peptidyl-tRNA binding site) A site (Aminoacyl- tRNA binding site) E site (Exit site) E P A Large subunit mRNA Small binding site subunit (b) Schematic model showing binding sites Growing polypeptide Amino end Next amino acid to be added to polypeptide chain E tRNA mRNA 3 5 Codons (c) Schematic model with mRNA and tRNA • A ribosome has three binding sites for tRNA: – The A site holds the tRNA that carries the next amino acid to be added to the chain – The P site holds the tRNA that carries the growing polypeptide chain – The E site is the exit site, where discharged tRNAs leave the ribosome 7
Translation Stage 1: Ribosome Association and Initiation of Translation • The initiation stage of translation brings together mRNA, a tRNA with the first amino acid, and the two ribosomal subunits • First, a small ribosomal subunit binds with mRNA and a special initiator tRNA • Then the small subunit moves along the mRNA until it reaches the start codon on mRNA (5’ - AUG- 3’) • Proteins called initiation factors bring in the large subunit that completes the translation initiation complex Fig. 17-17 Large ribosomal 3 5 U C subunit A P site 5 3 A G U Initiator GTP GDP tRNA E A mRNA 5 5 3 3 Start codon Small ribosomal Translation initiation complex mRNA binding site subunit 8
Translation Stage 2: Elongation of the Polypeptide Chain • During the elongation stage, amino acids are added one by one to the preceding amino acid • Each addition involves proteins called elongation factors and occurs in three steps: codon recognition, peptide bond formation, and translocation Fig. 17-18-1 Amino end of polypeptide E 3 mRNA site A P 5 site 9
Fig. 17-18-2 Amino end of polypeptide E 3 mRNA P site A 5 site GTP GDP E P A Fig. 17-18-3 Amino end of polypeptide E 3 mRNA site A P 5 site GTP GDP E P A E P A 10
Fig. 17-18-4 Amino end of polypeptide E 3 mRNA P site A Ribosome ready for 5 site next aminoacyl tRNA GTP GDP E E P A P A GDP GTP E P A Translation Stage 3: Termination of Translation • Termination occurs when a stop codon in the mRNA reaches the A site of the ribosome • The A site accepts a protein called a release factor • The release factor causes the addition of a water molecule instead of an amino acid • This reaction releases the polypeptide, and the translation assembly then comes apart 11
Fig. 17-19-1 Release factor 3 5 Stop codon (UAG, UAA, or UGA) Fig. 17-19-2 Release Free factor polypeptide 3 3 2 5 5 GTP Stop codon 2 GDP (UAG, UAA, or UGA) 12
Fig. 17-19-3 Release Free factor polypeptide 5 3 3 3 2 5 5 GTP Stop codon 2 GDP (UAG, UAA, or UGA) Polyribosomes • A number of ribosomes can translate a single mRNA simultaneously, forming a polyribosome (or polysome ) • Polyribosomes enable a cell to make many copies of a polypeptide very quickly 13
Fig. 17-20 Completed polypeptide Growing polypeptides Incoming ribosomal subunits Start of End of mRNA (5 end) mRNA (3 end) (a) Ribosomes mRNA (b) 0.1 µm Protein Folding and Post-Translational Modifications • During and after synthesis, a polypeptide chain spontaneously coils and folds into its three- dimensional shape • Proteins may also require post-translational modifications before doing their job • Some polypeptides are activated by enzymes that cleave them • Other polypeptides come together to form the subunits of a protein 14
Targeting Polypeptides to Specific Locations • Two populations of ribosomes are evident in cells: free ribsomes (in the cytoplasm) and bound ribosomes (attached to the ER) • Free ribosomes mostly synthesize proteins that function in the cytoplasm • Bound ribosomes make proteins of the endomembrane system and proteins that are secreted from the cell • Ribosomes are identical and can switch from free to bound • Polypeptide synthesis always begins in the cytosol • Synthesis finishes in the cytosol unless the polypeptide signals the ribosome to attach to the ER • Polypeptides destined for the ER or for secretion are marked by a signal peptide 15
• A signal-recognition particle (SRP) binds to the signal peptide • The SRP brings the signal peptide and its ribosome to the ER Fig. 17-21 Ribosome mRNA Signal peptide ER membrane Signal peptide Signal- Protein recognition removed particle (SRP) CYTOSOL Translocation complex ER LUMEN SRP receptor protein What happens to a protein when the cell doesn’t need it anymore? (We’ll talk more about this later.) 16
Recommend
More recommend