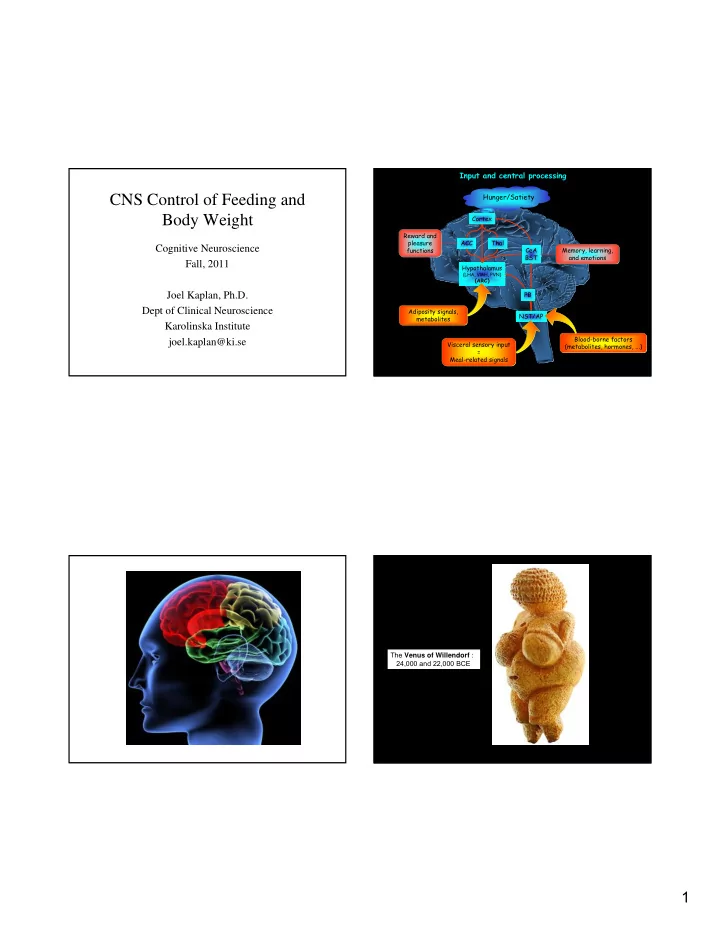

Input and central processing Hunger/Satiety CNS Control of Feeding and Body Weight Cortex Reward and pleasure ACC Thal Cognitive Neuroscience CeA Memory, learning, functions BST and emotions Fall, 2011 Hypothalamus (LHA, VMH, PVN) (ARC) PB Joel Kaplan, Ph.D. Dept of Clinical Neuroscience Adiposity signals, NST/AP metabolites Karolinska Institute Blood-borne factors joel.kaplan@ki.se Visceral sensory input (metabolites, hormones, ...) = Meal-related signals The Venus of Willendorf : 24,000 and 22,000 BCE 1
Obesity Trends* Am ong U.S. Adults BRFSS, 1 9 9 0 , 2 0 0 0 , 2 0 1 0 ( * BMI 3 0, or about 30 lbs. overw eight for 5’4” person) 2 0 0 0 1 9 9 0 From short- to long-term control of food intake and body weight. Perspective: “The Obesity Epidemic” 2 0 1 0 Body Mass Index: BMI = weight (kg) / height (m) 2 No Data <10% 10%–14% 15%–19% 20%–24% 25%–29% ≥ 30% BMI Classification < 18.5 underweight 18.5–24.9 normal weight 25.0–29.9 overweight Silhouettes and waist circumferences representing 30.0–34.9 class I obesity normal, overweight, and obese 35.0–39.9 class II obesity ≥ 40.0 class III obesity Neovius M, Teixeira ‐ Pinto A, Rasmussen F. Shift in the Composition of Obesity in Young Adults between 1969 and 2005. Int J Obes (Lond) 2007;Epub ahead of print (PMID: 18087264 ). 2
Losing Weight is Difficult 3
Fraternal Twins Genetics Play a Big Part Identical Twins 4
Evidence for a physiological Evidence for a physiological control of body weight: control of body weight: The body weight of adult individuals remains surprisingly constant over long periods of time If body weight in adult individuals (and thus body fat) is forced away from its normal level, compensatory changes in food intake and energy expenditure are induced 5
Recovery of rats‘ body weight after a period of caloric restriction (Keesey and Hirvonen, J. Nutr. 127:1875S-1883S, 1997) 6
Caloric homeostasis CNS Effectors of Energy Balance Energy Energy = intake expenditure 7
Human Obesity: Leptin Protein & MC4-R Mutations Human Obesity: Leptin Protein & MC4-R Mutations A comparison of a mouse unable to produce leptin thus resulting in obesity (left) and a normal mouse (right) 8
Energy Balance is Achieved Through Controls on Energy Intake [Feeding] and Energy Expenditure break Schwartz et al. 2002 Bachman et al. 2002 Enhancement of the direct inhibitory controls of meal size by adiposity signals Small meal Eating - Direct controls of meal size (e.g. CCK) Insulin Leptin Adipose tissue 9
Next 3 slides: Indirect Controls of Meal Size Modeling how direct controls (excitatory and inhibitory) interact to determine progress of individual meals, and Definition: Everything that is not a direct control cumulative intake (= meal size Categories Examples . Metabolic Changes in status of fat mass (Leptin and Insulin signaling) Rhythmic Diurnal Thermal Environmental and fever Conditioned Preferences, Aversions Cognitive Social, and in humans, cultural and esthetic Ecologic Relative densities of predators and foods ”Direct Controls” of Meal Size : Adequate stimuli act directly on Feedback intensity Positive preabsorptive mucosa along the surface of the Negative feedback feedback GI tract, from mouth through the small intestine Two Categories: Taste (Excitatory) Gut : Satiety Signals (postingestive inhibition Meal from gastric and post-gastric sources; e.g., Vagal afferents Eating time signaling stomach stretch, CCK 10
“Sham Feeding” paradigm: nutritive fluid meal taken by rats with gastric fistulas. Large meals are taken when fluid drips out of the stomach (i.e., when post-ingestive inhibitory feedback is eliminated.) Each curve is a substance of different palatability Pre- and Post-Prandial Correlations (skipped in class, but briefly FYI… ) Part of a conversation about how mechanisms controlling intake over short Term (i.e. meal size controls) carry over to influence intermediate-term intake (i.e., meal patterning; daily intake). Data from freely feeding rat: Graph to right shows that size of one meal influences when the next meal will be initiated; Not shown, data from schedule-fed A+B = high palatability, C+D = intermediate palatability, E+F = low palatability rats indicate that the size of a given meal correlates negatively with amount Pairs (A,B; C,D; E,F) are substances of same palatability but differ in rate of clearance from consumed during the next meal (e.g., big breakfast smaller lunch). intestine 11
A Behavioral Strategy for Assumptions Meal Size Control 1. Obese individuals eat at a higher rate than do 1. Eat very slowly their lean counterparts. 2. Take small bites of food 2. The obese take excessively large meals. 3. Chew a lot 3. Prospects for a ’normalization’ of meal size 4. Pause often during the meal (put down the fork) would be improved if ingestion rate were treated as a target behavior in a clasic behavior-modification approach to obesity Questions 1. Does eating slowly really affect meal size in obese or normal-weight people, or in lab rats? 2. What are the physiological implications of the hypothesis linking ingestion rate and meal size? 3. Can competing hypothesis be framed in physiological terms? 12
“Your body has an internal satiety (fullness) mechanism. When you have eaten enough, the mechanism sends out signals saying ‘Enough is enough!’ We think this takes about 20 minutes, although this is a very complex process involving the stomach, hormones in the small intestine, and other factors.” Size = 5 gram Size = 15 gram Competing Hypotheses S #07 Meals end as a function of elapsed feeding time. Friendly to a Postgastric theory of satiety S #11 Meals end simply as a function of the amount consumed (i.e., meal size is a ”regulated” parameter). Friendlier to a Gastric Model 13
Ingestion rate Meal size 25 350 300 Possible outcomes: 20 grams g/min 250 15 200 150 10 100 1) The total number of licks emitted remains constant 5 50 0 0 Meal size is halved. 5 10 15 5 10 15 Bite size (g) Bite size (g) 2) Meal size remains constant across sessions Number of bites Meal duration 25 60 the number of licks emitted is doubled. 50 20 number 40 min 15 30 3) Neither of these two outcomes applies. 10 20 5 (Not possible for both outcomes to be correct.) 10 0 0 5 10 15 5 10 15 Bite size (g) Bite size (g) C A B Intake (ml) Lick Count Meal Duration (sec) INTAKE (ml) LICK COUNT MEAL DURATION (sec) 30 5000 1200 25 1000 4000 20 800 3000 15 600 2000 10 400 1000 5 200 0 0 0 4 8 4 8 4 8 4 8 4 4 8 8 Lick volume ( l) Lick volume ul) 14
Conclusions: Generalization Examples: 1. For a given set of background conditions (food, physiological status, history, etc.), the meal ends as a function of the amount ’Drop Size’ Challenge: consumed, and not in relation to elapsed feeding time. Different sugars at different concentrations Deprivation 2. Meal termination cannot be anticipated by following Preload behavioral or ingestion-rate trajectories. Ingestive behavior is Pharmacological treatments flexible, and can vary broadly in service of the meal-size goal. Other Challenges: 3. Meal termination reflects highly accurate monitoring of Meal Interruption cumulative intake. Constraints on burst/pause pattern 4. Rat and human data indicate that eating glowly, by whatever contortion, isn’t likely to reduce intake. -- ”Myth Busted.” 15
Recommend
More recommend