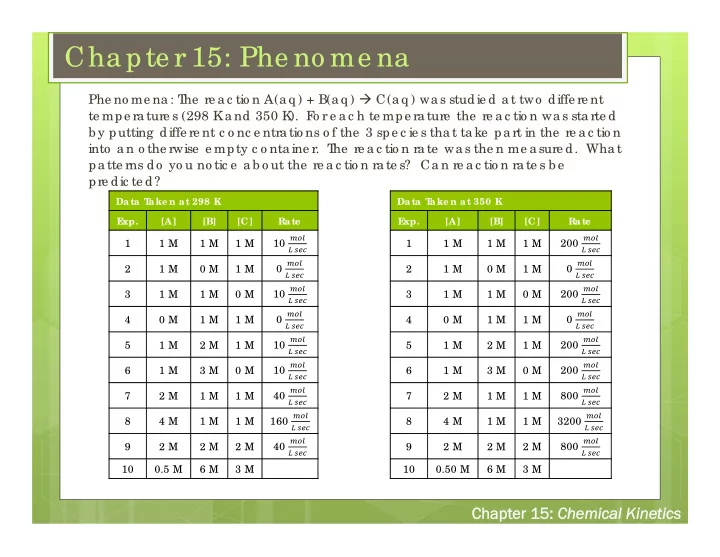

Cha pte r 15: Phe no me na he re a c tio n A(a q ) + B(a q ) C(a q ) wa s studie d a t two diffe re nt Phe no me na : T te mpe ra ture s (298 K a nd 350 K ). F o r e a c h te mpe ra ture the re a c tio n wa s sta rte d b y putting diffe re nt c o nc e ntra tio ns o f the 3 spe c ie s tha t ta ke pa rt in the re a c tio n into a n o the rwise e mpty c o nta ine r. T he re a c tio n ra te wa s the n me a sure d. Wha t pa tte rns do yo u no tic e a b o ut the re a c tio n ra te s? Ca n re a c tio n ra te s b e pre dic te d? Da ta T a ke n a t 298 K Da ta T a ke n a t 350 K E xp. [A] [B] [C] Ra te E xp. [A] [B] [C] Ra te ��� ��� 10 200 1 1 M 1 M 1 M 1 1 M 1 M 1 M � ��� � ��� ��� ��� 0 0 2 1 M 0 M 1 M 2 1 M 0 M 1 M � ��� � ��� ��� ��� 10 200 3 1 M 1 M 0 M 3 1 M 1 M 0 M � ��� � ��� ��� ��� 0 0 4 0 M 1 M 1 M 4 0 M 1 M 1 M � ��� � ��� ��� ��� 10 200 5 1 M 2 M 1 M 5 1 M 2 M 1 M � ��� � ��� ��� ��� 10 200 6 1 M 3 M 0 M 6 1 M 3 M 0 M � ��� � ��� ��� ��� 7 2 M 1 M 1 M 40 7 2 M 1 M 1 M 800 � ��� � ��� ��� ��� 8 4 M 1 M 1 M 160 8 4 M 1 M 1 M 3200 � ��� � ��� ��� ��� 9 2 M 2 M 2 M 40 9 2 M 2 M 2 M 800 � ��� � ��� 10 0.5 M 6 M 3 M 10 0.50 M 6 M 3 M Chapt Chapter 1 r 15: : Chemic Chemical Kine Kinetic tics
Cha pte r 15 Che mic a l K ine tic s Big Ide a : T he ra te s o f c he mic a l o T he rmo Re vie w re a c tio ns a re o Re a c tio n Ra te s de sc rib e d b y simple o Ra te L a ws e xpre ssio ns tha t a llo w o Co nc e ntra tio n a nd us to pre dic t the T ime c o mpo sitio n o f a re a c tio n mixture a t o Re a c tio n Me c ha nisms a nytime . T he se o E xpla ining Re a c tio n e xpre ssio ns a lso Ra te F a c to rs sug g e st the ste ps in whic h the re a c tio ns ta ke s pla c e . 2
Re a c tio n Ra te s Ca ta lyst: A sub sta nc e tha t inc re a se s the re a c tio n ra te witho ut b e ing c o nsume d in the re a c tio n. Homog e ne ous Ca ta lyst: A c a ta lyst tha t is in the sa me pha se a s the re a c ta nts. He te rog e ne ous Ca ta lyst: A c a ta lyst tha t is in a diffe re nt pha se tha n the re a c ta nts. Chapt Chapter 1 r 15: : Chemic Chemical Kine Kinetic tics 3
Re a c tio n Ra te s Re a c tion Ra te s: T he c ha ng e in c o nc e ntra tio n o f o ne o f the re a c ta nts o r pro duc ts divide d b y the time inte rva l o ve r whic h the c ha ng e ta ke s pla c e . R P Ave rage Rate o f Ave rage Rate o f Co nsumptio n o f R: Pro duc tio n o f P: ���� � � ∆� ���� � ∆� ∆� ∆� No Note: Rates are always positive, therefore, since the reactants are consumed, a negative sign must be added to make the rate positive. U nique Ave rage Rate (U AR) ��� � � 1 ∆ � ∆� � � 1 ∆ � ∆� � 1 ∆ � � � � ∆� Chapter 1 Chapt r 15: : Chemic Chemical Kine Kinetic tics 4
Re a c tio n Ra te s Insta nta ne ous Ra te of Re a c tion T he b e st a ppro xima tio n to the ra te a t a sing le insta nt is o b ta ine d b y dra wing a line ta ng e nt to the plo t o f the c o nc e ntra tio n a g a inst time . T he slo pe o f the ta ng e nt line is c a lle d the insta nta ne o us ra te o f the re a c tio n. Chapt Chapter 1 r 15: : Chemic Chemical Kine Kinetic tics 5
Ra te L a ws a w: An e q ua tio n e xpre ssing the Ra te L insta nta ne o us re a c tio n ra te in te rms o f the c o nc e ntra tio ns, a t a ny insta nt, o f the sub sta nc e s ta king pa rt in the re a c tio n. ���� � � � � � � … No Note: k is the rate constant and x ,y, … are the orders of reaction. Note: This form of the rate law is called the differential rate law. No ��������� Note: The units of rate are always No ������ � ���� , therefore, the units of k will differ depending on the overall reaction order. Chapter 1 Chapt r 15: : Chemic Chemical Kine Kinetic tics 6
Ra te L a ws T hing s to know a bout the ra te la w: Ra te la ws c a n c o nta in pro duc ts, re a c ta nts, c a ta lysts b ut usua lly o nly sta rting ma te ria l. Ra te la ws do no t c o nta in inte rme dia te s. Ra te la ws c a n ONL Y b e de te rmine d e xpe rime nta lly. Orde rs do NOT c o rre la te with c o e ffic ie nts in b a la nc e d e q ua tio n! Orde rs c a n b e a n inte g e rs, ze ro s, fra c tio ns, po sitive s, OR ne g a tive s! E a c h spe c ie s ha s its o wn individua l re a c tio n o rde r. T he o ve ra ll re a c tio n o rde r is the sum o f the individua l o rde rs fo und in the re a c tio n. Chapt Chapter 1 r 15: : Chemic Chemical Kine Kinetic tics 7
Ra te L a ws How to find the units of k Ste p 1: De te rmine the o rde r o f the re a c tio n Examples Ex amples for rate=k[A][B]: Overall order 2 Ste p 2: Sub tra c t 1 fro m the o ve ra ll o rde r Examples Ex amples for rate=k[A][B]: 1 � Ste p 3 : F ind # ���� � ��� ·� � Examples Ex amples for rate=k[A][B]: 1 � � � · � ��� · � ��� � Chapter 1 Chapt r 15: : Chemic Chemical Kine Kinetic tics 8
Ra te L a ws 0 th 1 st 2 nd Orde r 0 2 4 6 [A] 0 10 20 30 0 10 20 30 0 10 20 30 time 0 0.3 0.6 0.9 Ra te Initia l Conc e n- 0 2 4 6 0 2 4 6 0 2 4 6 tra tion ���� � ���� � ���� � � ���� � ���� Ra te L a w Chapter 1 Chapt r 15: : Chemic Chemical Kine Kinetic tics 9
Ra te L a ws De te rmine the ra te la w: A + B + C 2D Initia l Ra te ( ��� E xpe rime nt [A] o (M) [B] o (M) [C] o (M) �·� ) 1 1 6 4 20 2 2 6 2 80 3 2 3 2 40 4 1 6 2 20 Ge ne ra l Ra te L a w: 1) Orde r with re spe c t to A: 2) Orde r with re spe c t to B: 3) Orde r with re spe c t to C: 4) Chapter 1 Chapt r 15: : Chemic Chemical Kine Kinetic tics 10
Ra te L a ws De te rmine the ra te la w a nd k fo r 2A + B 2C Initia l Ra te ( ��� E xpe rime nt [A] o (M) [B] o (M) �·� ) 1 0.050 0.10 0.074 2 0.10 0.20 0.888 3 0.050 0.20 0.222 Chapter 1 Chapt r 15: : Chemic Chemical Kine Kinetic tics 11
Ra te L a ws De te rmining Orde r (long wa y) Ste p 1: F ind two e xpe rime nts in whic h the c o nc e ntra tio ns o f e ve rything , e xc e pt o ne spe c ie s, is he ld c o nsta nt. Ste p 2: Divide the ra te la ws fo r the se two e xpe rime nts b y e a c h o the r. Note: This will cancel out k and all other variables except for the order that you No are trying to determine. � � � � � � � Math No Math Note: � Ste p 3: So lve fo r o rde r. Math No Math Note: It is sometimes useful to take the log of both sides of the equation. The log(x y )=ylog(x). Chapter 1 Chapt r 15: : Chemic Chemical Kine Kinetic tics 12
Ra te L a ws Student Question T he ra te la w fo r the fo llo wing re a c tio n 2NO(g ) +O 2 (g ) 2NO 2 (g ) wa s e xpe rime nta lly fo und to b e in the fo rm ra te =k[NO] x [O 2 ] y I t wa s a lso fo und tha t whe n the NO c o nc e ntra tio n wa s do ub le d, the ra te o f the re a c tio n inc re a se s b y a fa c to r o f 4. I n a dditio n, whe n b o th the O 2 a nd the NO c o nc e ntra tio n we re do ub le d, the ra te inc re a se s b y a fa c to r o f 8. Wha t is the re a c tio n o rde r o f O 2 ? a ) 0 th b ) 1 st c ) 2 nd d) 3 rd e ) No ne o f the a b o ve Chapt Chapter 1 r 15: : Chemic Chemical Kine Kinetic tics 13
Ra te L a ws De te rmine the ra te la w a nd k fo r A + B 2C Initia l Ra te ( ��� E xpe rime nt [A] o (M) [B] o (M) �·� ) 1 5.0 0.010 5.0 2 5.0 0.020 10. Pse udo Orde r Re a c tion: A re a c tio n in whic h the ra te la w c a n b e simplifie d b e c a use a ll b ut o ne o f the spe c ie s ha ve virtua lly c o nsta nt c o nc e ntra tio ns. Chapt Chapter 1 r 15: : Chemic Chemical Kine Kinetic tics 14
Co nc e ntra tio n a nd T ime Ze ro Orde r Inte g ra te d Ra te L a w Rate 1 ) k dx x const dA 2 ) k 5 ) A A kt dt 3 ) dA kdt A kt A A t 4 ) dA k dt 0 A Chapter 1 Chapt r 15: : Chemic Chemical Kine Kinetic tics 15
Co nc e ntra tio n a nd T ime F irst Orde r Inte g ra te d Ra te L a w 1 Rate 1 ) k A ln dx x const x dA 2 ) k A 5 ) ln ln A A kt dt 1 3 ) dA kdt A ln ln A kt A 1 A t 4 ) dA k dt 0 A A Chapter 1 Chapt r 15: : Chemic Chemical Kine Kinetic tics 16
Recommend
More recommend