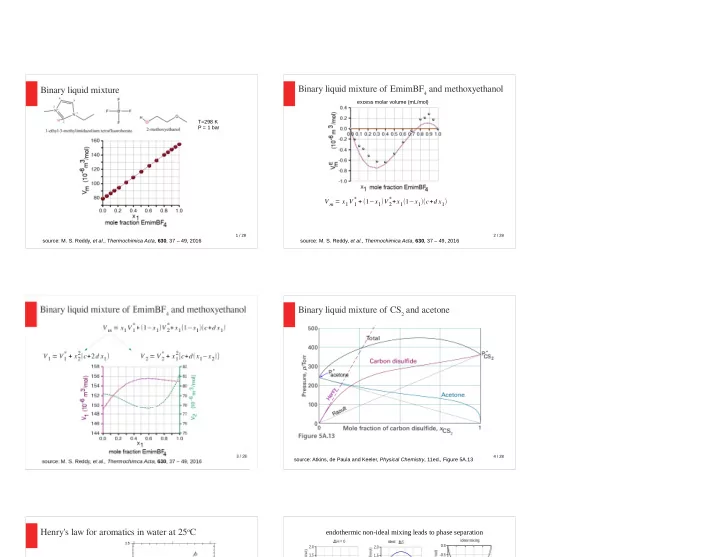

Binary liquid mixture of EmimBF 4 and methoxyethanol Binary liquid mixture excess molar volume (mL/mol) T=298 K P = 1 bar * + ( 1 − x 1 ) V 2 * + x 1 ( 1 − x 1 )( c + d x 1 ) V m = x 1 V 1 1 / 28 2 / 28 source: M. S. Reddy, et al ., Thermochimica Acta , 630 , 37 – 49, 2016 source: M. S. Reddy, et al ., Thermochimica Acta , 630 , 37 – 49, 2016 Binary liquid mixture of CS 2 and acetone 4 / 28 source: Atkins, de Paula and Keeler, Physical Chemistry , 11ed., Figure 5A.13 Henry's law for aromatics in water at 25 o C endothermic non-ideal mixing leads to phase separation
Molecular mass from osmotic pressure chap. 5 sect. C. binary vapor-liquid equilibrium triethylene glycol in water at 25 o C ethyl lactate and ethanol T = 40 o C line: 0.168 + 3.5X10 -5 data from http://lpsb.nichd.nih.gov/tri_glycol.htm source: Dung T. Vu, et al ., J. Chem. Eng. Data , 2006 , 51 , 1220-1225. chap. 5 sect. C. binary vapor-liquid equilibrium mixtures of nitrogen and oxygen are nearly ideal at fixed P at fixed T source: International Critical Tables source: just a sketch source: Dung T. Vu, et al ., J. Chem. Eng. Data , 2006 , 51 , 1220-1225. negative deviation yields high-boiling azeotrope nitrogen and oxygen at 1 atm a nearly-ideal mixture
positive deviation from ideality yields a low-boiling azeotrope less-than-ideal liquid-vapor equilibrium methanol and diethyl ether at T=303.15K Distillation cannot purify beyond the azeotrope. For example, ethanol and water cannot be distilled to greater than 96% ethanol. Source: The Phase Rule and Phase Relations , Baudilio Coto, et al., Journal of the Chemical Society Faraday Transactions , 91 (24), 4381-4388, 1995. by Sydney T. Bowden, Macmillan & Co., 1950 less-than-ideal liquid-vapor equilibrium less-than-ideal liquid-vapor equilibrium methanol and diethyl ether at T=303.15K methanol and diethyl ether at T=303.15K At 50 kPa, At 50 kPa, Using the lever rule: If x methanol =0.600, then the y vapor = 0.407 y vapor = 0.407 fraction of all moles that are in the liquid phase is and and n liquid (0.600 - 0.407) x liquid = 0.855 x liquid = 0.855 = = 0.431 n (0.855 - 0.407) Baudilio Coto, et al., Journal of the Chemical Society Baudilio Coto, et al., Journal of the Chemical Society Faraday Transactions , 91 (24), 4381-4388, 1995. Faraday Transactions , 91 (24), 4381-4388, 1995. 5D. binary solid-liquid phase diagrams the case of no solid solution eutectic point
5D. binary solid-liquid phase diagrams the case of a compound that melts congruently congruent melting eutectic point 303K is the melting point of Gallium source: I. Ansara, et al ., Calphad , 18(2), 177-222, 1994 . Figure 12. 19 / 28 DOI: 10.2475/ajs.s4-40.236.161 ternary phase diagram ternary phase diagram polypropylene polyol (PPP) and water and methanol polypropylene polyol (PPP) and water and methanol Source: C. H. Lee, et al., Polymer , 146, 169-178, 2018 Source: C. H. Lee, et al., Polymer , 146, 169-178, 2018 5F. Debye-Huckel limiting law (DHLL) 5F. Debye-Huckel limiting law
Recommend
More recommend