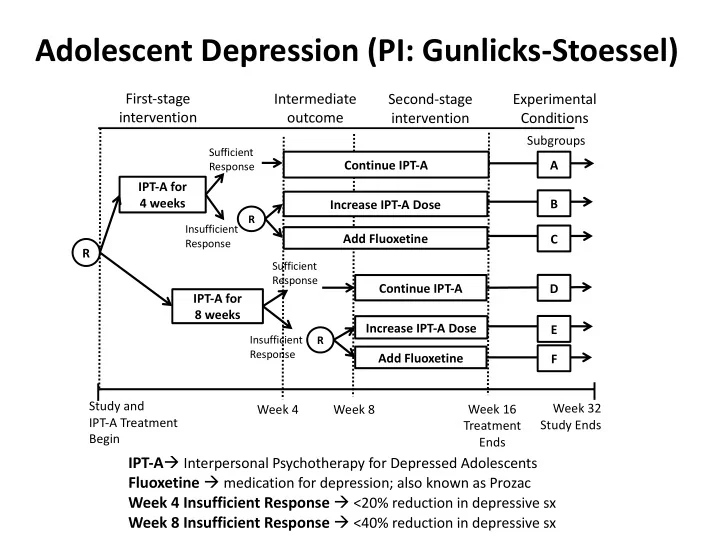

Adolescent Depression (PI: Gunlicks-Stoessel) First-stage Intermediate Second-stage Experimental intervention outcome intervention Conditions Subgroups Sufficient Continue IPT-A A Response IPT-A for 4 weeks B Increase IPT-A Dose R Insufficient Add Fluoxetine C Response R Sufficient Response Continue IPT-A D IPT-A for 8 weeks Increase IPT-A Dose E Insufficient R Response Add Fluoxetine F Study and Week 32 Week 4 Week 8 Week 16 IPT-A Treatment Study Ends Treatment Begin Ends IPT-A à Interpersonal Psychotherapy for Depressed Adolescents Fluoxetine à medication for depression; also known as Prozac Week 4 Insufficient Response à <20% reduction in depressive sx Week 8 Insufficient Response à <40% reduction in depressive sx
Autism (PI: Kasari) First-stage Intermediate Second-stage Experimental intervention outcome intervention Conditions Subgroups Early JASPER + EMT A Response JASPER + Intensified EMT B JASPER + EMT R Slow Response R C JASPER + EMT + SGD Early JASPER + EMT + SGD D Response JASPER + EMT + SGD Slow Intensified E Response JASPER + EMT + SGD Study and Week 12 Month 6 Treatment Begin JASPER à Joint Attention and joint Engagement EMT à Enhanced Milieu Teaching SGD à Speech Generating Device (e.g., an iPad or Dynavox) Early Response à 25%+ improvement on 7+ communication measures
Child ADHD (PI: Pelham) First-stage Intermediate Second-stage Experimental intervention outcome intervention Conditions Subgroups Response Continue A MED + Monitoring Augment (MED+BMOD) B R Non- Intensify MED C Response R Response Continue D BMOD + Monitoring Augment (BMOD+MED) E Non- R Response Intensify BMOD F Beginning of school End of school year Monthly, starting year at Week 8 MED à Low-dose methylphenidate (am) Intensify MED à Higher-dose or add pm BMOD à Behavioral Modification Intensify BMOD à Intensify/add components Non-response à Teacher-rated Individualized Target Behavior Evals < 75% and Teacher-rated Impairment Rating Scale > 3 on at least one domain
Recommend
More recommend