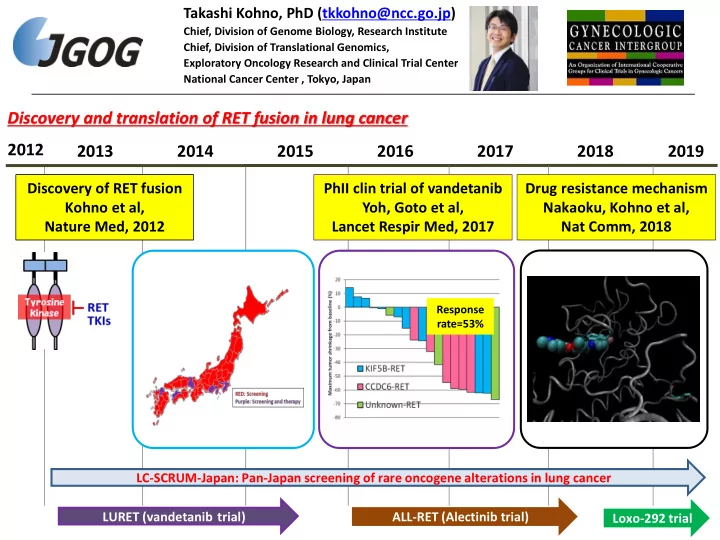

Takashi Kohno, PhD (tkkohno@ncc.go.jp) Chief, Division of Genome Biology, Research Institute Chief, Division of Translational Genomics, Exploratory Oncology Research and Clinical Trial Center National Cancer Center , Tokyo, Japan Discovery and translation of RET fusion in lung cancer 2012 2013 2014 2015 2016 2017 2018 2019 Discovery of RET fusion PhII clin trial of vandetanib Drug resistance mechanism Kohno et al, Yoh, Goto et al, Nakaoku, Kohno et al, Nature Med, 2012 Lancet Respir Med, 2017 Nat Comm, 2018 Response rate=53% LC-SCRUM-Japan: Pan-Japan screening of rare oncogene alterations in lung cancer LURET (vandetanib trial) ALL-RET (Alectinib trial) Loxo-292 trial 1
Implementation of NCC Oncopanel Test in Japan NGS test for 114 genes Somatic mutation FFPE tumor sample & Tumor board Clinical trial Germline mutation IC Peripheral blood meeting Off-label use Tumor mutation burden 114 mutation ・ amplification (whole exon) 13 fusion ABL1 CRKL IDH2 NF1 RAC2 ALK ACTN4 CREBBP IGF1R NFE2L2 RAD51C AKT2 AKT1 CTNNB1/b-catenin IGF2 NOTCH1 RAF1/CRAF AKT3 AKT2 CUL3 IL7R NOTCH2 RB1 BRAF AKT3 DDR2 JAK1 NOTCH3 RET ERBB4 ALK EGFR JAK2 NRAS RHOA FGFR2 NRG1 APC ENO1 JAK3 ROS1 FGFR3 ARAF EP300 KDM6A/UTX NTRK1 SETBP1 NRG1 ARID1A ERBB2/HER2 KEAP1 NTRK2 SETD2 NTRK1 ARID2 ERBB3 KIT NTRK3 SMAD4 NTRK2 ATM ERBB4 KRAS NT5C2 SMARCA4/BRG1 PDGFRA AXIN1 ESR1/ER MAP2K1/MEK1 PALB2 SMARCB1 RET AXL EZH2 MAP2K2/MEK2 PBRM1 SMO ROS1 BAP1 FBXW7 MAP2K4 PDGFRA STAT3 BARD1 FGFR1 MAP3K1 PDGFRB STK11/LKB1 BCL2L11 FGFR2 MAP3K4 PIK3CA TP53 BRAF FGFR3 MDM2 PIK3R1 TSC1 Japanese FDA approval in Dec, 2018 BRCA1 FGFR4 MDM4 PIK3R2 VHL BRCA2 FLT3 MET POLD1 Reimbursement by National insurance system CCND1 GNA11 MLH1 POLE CD274/PD-L1 GNAQ MTOR PRKCI will start in June, 2019 CDK4 GNAS MSH2 PTCH1 2 CDKN2A HRAS MYC PTEN CHEK2 IDH1 MYCN RAC1
Precision Medicine for Ovarian Cancer “ Cancer ” with GOF Mutation “Cancer” with LOF Mutation → Targeting Synthetic Lethal Partner → Directly Targeting Oncoprotein GOF: Gain of Function LOF: Loss of Function Normal Cells Cancer Cells Normal Cells Cancer Cells A B a a A B B B Survival Lethal Survival (Synthetic) Lethal a: BRCA1/BRCA2 a: EGFR Gefitinib etc Ovary Synthetic lethality Lung Breast B: PARP1 Olaparib etc a: BRAF Vemurafenib Skin a: ARID1A a: ALK Crizotinib etc Ovary Synthetic lethality Lung B: GSH APR-246 a: RET Vandetanib etc 3 Lung
Targeting the Vulnerability of Glutathione Metabolism in ARID1A -Deficient Cancers (Ogiwara H, Takahashi K, …., Okamoto A, Kohno T. Cancer Cell , 2019) RMG-I:ARID1A + TOV21G:ARID1A ー RMG-I Xenograft Tumor Volume (mm 3 ) TOV21G Xenograft 800 Tumor Volume (mm 3 ) 1500 APR-246- APR-246 - APR-246+ 600 APR-246 + 1000 400 500 ** 200 *** *** ** ** ** 0 0 4 0 5 10 15 20 0 2 4 6 8 10 12 Days Days
Ovarian clear cell carcinoma frequently (50%) shows ARID1A deficiency ARID1A maintains Glutathione (GSH) homeostasis by enhancing SLC7A11 transcription Low SLC7A11 expression causes low basal GSH levels in ARID1A-deficient cancer cells Inhibiting GSH by APR-246 in ARID1A-deficient cancer cells causes apoptosis by ROS RMG-I:ARID1A + TOV21G:ARID1A ー RMG-I Xenograft Tumor Volume (mm 3 ) TOV21G Xenograft 800 Tumor Volume (mm 3 ) 1500 APR-246- APR-246 - APR-246+ 600 APR-246 + 1000 400 500 ** 200 *** *** ** ** ** 0 0 5 0 5 10 15 20 0 2 4 6 8 10 12 Days Days
ARID1A-deficient Ovarian Cancer Cells Lack SLC7A11 Expression anti-ARID1A (HPA005456, 1:2000 dilution; Sigma-Aldrich) anti-SLC7A11 (ab175186, 1:400 dilution; Abcam) 6
APR-246 (APREA Therapeutics) Inactivates Glutathione (GSH) TOV21G: ARID1A(-), TP53(WT) OCCC p53 activation MQ GSH inactivation TrxR (Bykov VJN et al., 2016) P53 mutated ARID1A wild type APR-246 clinical trials 7 https://www.aprea.com/pipeline/apr-246/
Proposal of APR-246 (APREA Therapeutics) Trial APR246 treatment Global clinical trial? for ovarian clear cell carcinoma (and endometrioid ca? ) Coupling research ARID1A (+) ARID1A (-) ・ Relationship between response & ARID1A/SLC7A11expression (by IHC) SLC7A11 (+) SLC7A11 (-) ・ Monitoring cfDNA for ARID1A mutation (by Guardant360 test?) Non-responder? Responder? 8
Monitoring cfDNA for ARID1A mutation (by Guardant360 test?) Guardant 360 9
Comprehensive alliance between The JikeiUniversity & National Cancer Center No. of Global Trials in NCCH Types of Global Trials in NCCH 450 Domestic IIT 198 200 Global 4 400 3 Others 365 Total 169 350 2 P-III 322 3 145 293 150 P-II 278 300 1 4 110 2 … P-I/II 115 250 1 102 103 206 4 P-I 1 100 5 200 94 83 . 5 1 150 73 28 67 23 100 50 198 58 20 169 167 163 154 148 145 143 21 123 14 18 115 50 103 14 83 11 9 33 12 6 21 14 14 4 10 0 3 0 10 2012 2013 2014 2015 2016 2017 2012 2013 2014 2015 2016 2017
Takashi Kohno, PhD (tkkohno@ncc.go.jp) National Cancer Center , Tokyo, Japan Lancet Oncology, 2017 Dr. Kenji Tamura (a PI for early phase clinical trials) Chairman, Department of Breast and Medical Oncology, National Cancer Center Hospital Director, Outpatient Treatment Center E-mail: ketamura@ncc.go.jp 11
Recommend
More recommend