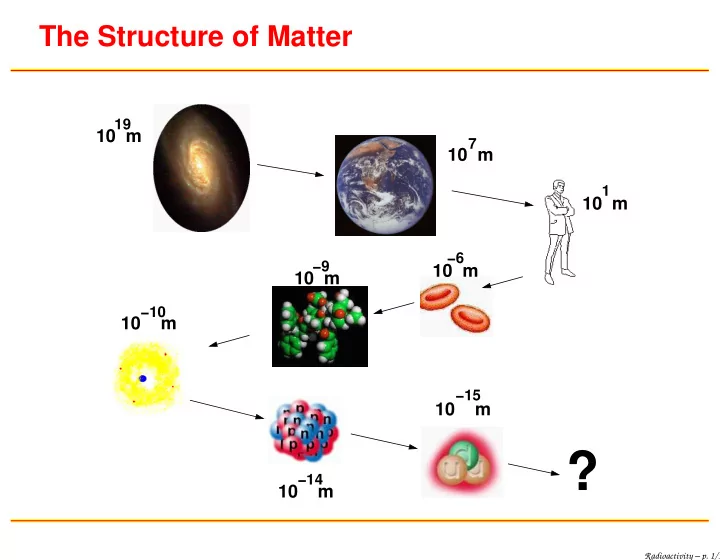

The Structure of Matter 19 10 m 7 10 m 1 10 m −6 −9 10 m 10 m −10 10 m −15 10 m ? −14 10 m Radioactivity – p. 1/1
Radioactivity and Nuclear Decay At the end of the nineteenth century Henri Becquerel discovers the spon- taneous emission of ‘rays’. The surprise was that no energy in- put was required. These rays carry off huge amounts of energy. Some examples of ‘rays’. Original Photographic Plate Developed by Henri Becquerel. 212 208 4 Pb + He (α) Po Pb + He 84 82 2 212 Bi 212 (β) + ν (undetected) Po + e e 83 84 137 137 + ν (undetected) Cs Ba(0.662 keV) + e 55 56 e 137 γ Ba(0.0 keV) + 56 Radioactivity – p. 2/1
The 4 He − 234 90 Th Potential 40 20 0 V � MeV � Blue � Known � 20 Red � AGuess � 40 0 10 20 30 40 50 60 70 r � fm � Radioactivity – p. 3/1
Rutherford Scattering ZnS Microscope Collimator Alpha source Scattered helium Alpha beam Thorium target 210 4 206 Po He + Pb 84 2 82 Radioactivity – p. 4/1
The 4 He − 234 90 Th Potential 40 20 0 V � MeV � Blue � Known � 20 Red � AGuess � 40 0 10 20 30 40 50 60 70 r � fm � Radioactivity – p. 5/1
Milking the Cow This ‘clock’ ticks by producing a short-lived, radioactive material. 137 Cs − Start with a liquid containing the ra- e 55 dioactive isotope 137 Cs that decays excited state very slowly. (0.662 MeV) γ 137 Cs → e − + 137 Ba(0 . 662 MeV) ground state 137 The number “0.662 MeV” means 56 Ba there is still energy (0.662 MeV) Decay scheme of stored in the Ba-137 nucleus. cesium-137. The excited Ba-137 then emits a high-energy photon or gamma ray to reach the stable ground state of 137 Ba . 137 Ba(0 . 662) → 137 Ba(0 . 0)+ γ Radioactivity – p. 6/1
Using the Reduced χ 2 The χ 2 and reduced χ 2 are defined as N (( y i − f ( x i )) 2 χ 2 = � σ 2 i i =1 and χ 2 reduced χ 2 = N − d.o.f where N is the number of data points. R. Muto, et al. , Phys. Rev. Lett., 98 , 042501 (2007). Radioactivity – p. 7/1
Geiger -Muller Tube A Geiger-Muller tube (or GM tube) is the sensing element of a Geiger counter instrument that can detect a single particle of ionizing radiation. It is a type of gaseous ionization detector with an operating voltage in the Geiger plateau. Radioactivity – p. 8/1
Poisson Statistics P ( m : n, p ) = 1 m ! µ m e − µ µ = np m - no. of events µ - average n - no. of trials p - probability of an event Probability of a discrete event occurring m times in a particular time period. Radioactivity – p. 9/1
Poisson Statistics P ( m : n, p ) = 1 m ! µ m e − µ µ = np m - no. of events µ - average n - no. of trials p - probability of an event Probability of a discrete event occurring m times in a particular time period. Number of soldiers killed by horse-kicks each year in Prussian cavalry corp (famous example in by a book of Ladislaus Josephovich Bortkiewicz (1868-1931)). Number of yeast cells for brewing Guinness (William Sealy Gosset (1876-1937)). The number of phone calls arriving at a call center per minute. The number of deaths per year in a given age group. The number of jumps in a stock price in a given time interval. The number of mutations in a given stretch of DNA after a certain amount of radiation. The proportion of cells that will be infected at a given multiplicity of infection. Radioactivity – p. 9/1
How Old is the Shroud of Turin? Radioactivity – p. 10/1
Radiocarbon Dating Cosmic ray proton 14 N Atmosphere neutron proton 14 N 14 C O 2 14 CO 2 dead or buried material 14 loses C Radioactivity – p. 11/1
Radiocarbon Dating Radioactivity – p. 12/1
Radiocarbon Dating Radioactivity – p. 12/1
Radiocarbon Calibration Curve Radioactivity – p. 13/1
Recommend
More recommend