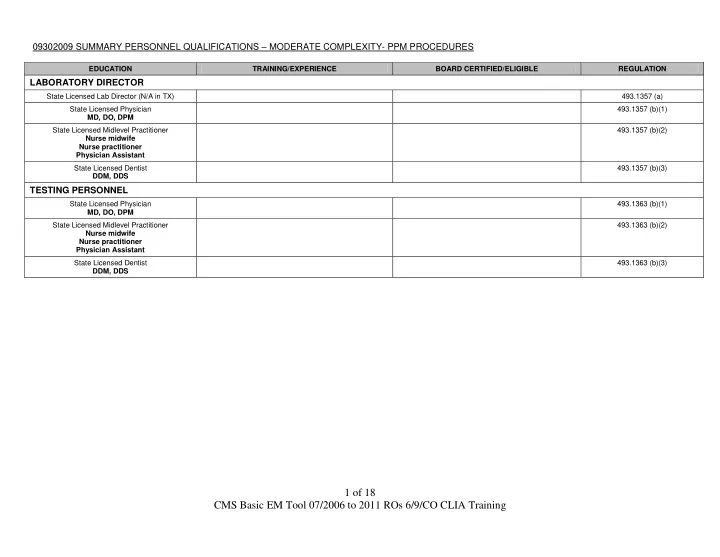

09302009 SUMMARY PERSONNEL QUALIFICATIONS – MODERATE COMPLEXITY- PPM PROCEDURES EDUCATION TRAINING/EXPERIENCE BOARD CERTIFIED/ELIGIBLE REGULATION LABORATORY DIRECTOR State Licensed Lab Director (N/A in TX) 493.1357 (a) State Licensed Physician 493.1357 (b)(1) MD, DO, DPM State Licensed Midlevel Practitioner 493.1357 (b)(2) Nurse midwife Nurse practitioner Physician Assistant State Licensed Dentist 493.1357 (b)(3) DDM, DDS TESTING PERSONNEL State Licensed Physician 493.1363 (b)(1) MD, DO, DPM State Licensed Midlevel Practitioner 493.1363 (b)(2) Nurse midwife Nurse practitioner Physician Assistant State Licensed Dentist 493.1363 (b)(3) DDM, DDS 1 of 18 CMS Basic EM Tool 07/2006 to 2011 ROs 6/9/CO CLIA Training
09302009 SUMMARY PERSONNEL QUALIFICATIONS – MODERATE COMPLEXITY EDUCATION TRAINING/EXPERIENCE BOARD CERTIFIED/ELIGIBLE REGULATION LABORATORY DIRECTOR State Licensed Lab Director (N/A in TX) N/A Certified in Anatomic or Clinical Pathology or both 493.1405 (b)(1)(ii) AND by the American Board of Pathology or the American Osteopathic Board of Pathology OR board eligible State Licensed Physician MD, DO 1 year directing or supervising non-waived testing N/A 493.1405 (b)(2)(ii)(A) OR Effective 09/01/93 , have at least 20 CME in laboratory N/A 493.1405 (b)(2)(ii)(B) practice defined in 493.1407 OR Laboratory training during medical residency equivalent N/A 493.1405 (b)(2)(ii)(C) to 493.1405 (b)(2)(ii)(B) PhD : chemical, physical, biological, or clinical N/A Certified by: American Board of Medical Microbiology, 493.1405 (b)(3)(i) laboratory science the American Board of Clinical Chemistry, the American Board of Bioanalysis, or the American Board of Medical Laboratory Immunology 1 year directing or supervising non-waived testing N/A 493.1405 (b)(3)(ii) MASTER’S : chemical, physical, biological, 1 year laboratory training or experience in non-waived N/A 493.1405 (b)(4)(iii) clinical laboratory science, or medical technology testing AND 1 year of supervisory laboratory experience in non- waived testing BACHELOR’S : chemical, physical, biological, 2 years laboratory training or experience in non-waived N/A 493.1405 (b)(5)(iii) clinical laboratory science, or medical technology testing AND 2 years of supervisory laboratory experience in non- waived testing Be serving as a Laboratory Director and have N/A N/A 493.1405 (b)(6) been previously qualified or could have qualified as a LD on or before 02/28/92 under 493.1406. 2 of 18 CMS Basic EM Tool 07/2006 to 2011 ROs 6/9/CO CLIA Training
09302009 SUMMARY PERSONNEL QUALIFICATIONS – MODERATE COMPLEXITY 493.1406 LABORATORY DIRECTOR QUALIFICATION ON OR BEFORE FEBRUARY 28, 1992 EDUCATION TRAINING/EXPERIENCE BOARD CERTIFIED/ELIGIBLE REGULATION LABORATORY DIRECTOR State Licensed Lab Director (N/A in TX) N/A Certified in Anatomic or Clinical Pathology or both 493.1406 (b)(1) AND by the American Board of Pathology or the American State Licensed Physician Osteopathic Board of Pathology OR board eligible MD, DO Certified in at least one of the laboratory 493.1406 (b)(2)(i) specialties by the American Board of Pathology or American Osteopathic Board of Pathology Certified by the American Board of Medical 493.1406 (b)(2)(ii) Microbiology, the American Board of Clinical Chemistry, the American Board of Bioanalysis, OR other national accrediting board in one of the laboratory specialties Certified by the American Society of Cytology OR 493.1406 (b)(2)(iii) board eligible Subsequent to graduation, has had 4 or more years full N/A 493.1406 (b)(2)(iv) time general laboratory training and experience of which at least 2 years were spent acquiring proficiency in one of the laboratory specialties DDS (Licensed) N/A ORAL PATHOLOGY ONLY 493.1406 (b)(3) Certified by the American Board of Oral Pathology, American Board of Pathology , or the American Osteopathic Board of Pathology OR board eligible PhD: chemical, physical, or biological science N/A Certified by: American Board of Medical Microbiology, 493.1406 (b)(4)(i) the American Board of Clinical Chemistry, the American Board of Bioanalysis, or the American Board of Medical Laboratory Immunology Subsequent to graduation, has had 4 or more years full N/A 493.1406 (b)(4)(ii) time general laboratory training and experience of which at least 2 years were spent acquiring proficiency in one of the laboratory specialties EDUCATION TRAINING/EXPERIENCE REGULATION With respect to individuals first qualifying before Licensed MD Subsequent to graduation had 4 years of pertinent full 493.1406 (b)(5)(i) 07/01/71 , had been responsible for the direction time laboratory experience of a laboratory for 12 months between 07/01/61 MASTER’S : chemical, physical, or biological sciences Subsequent to graduation had 4 years of pertinent full 493.1406 (b)(5)(ii) and 01/01/68 and time laboratory experience BACHELOR’S : chemical, physical, or biological science Subsequent to graduation had 6 years of pertinent full 493.1406 (b)(5)(iii) time laboratory experience 3 of 18 CMS Basic EM Tool 07/2006 to 2011 ROs 6/9/CO CLIA Training
09302009 SUMMARY PERSONNEL QUALIFICATIONS – MODERATE COMPLEXITY Continued, 493.1406 LABORATORY DIRECTOR QUALIFICATION ON OR BEFORE FEBRUARY 28, 1992 EDUCATION TRAINING/EXPERIENCE BOARD CERTIFIED/ELIGIBLE REGULATION Achieved satisfactory grade on PHS examination on N/A 493.1406 (b)(5)(iv) or before 07/01/70 Qualify under State law to direct a laboratory in that State N/A 493.1406 (b)(6) TECHNICAL CONSULTANT State Licensed Technical Consultant (N/A in TX) N/A Certified in Anatomic or Clinical Pathology or both 493.1411 (b)(1)(ii) AND by the American Board of Pathology or the American Osteopathic Board of Pathology OR board eligible State Licensed Physician MD, DO, DPM 1 year laboratory training or experience in the designated N/A 493.1411 (b)(2)(ii) specialty/subspecialty for which TC is responsible PhD, MASTER’S : chemical, physical, biological, 1 year laboratory training or experience in the designated N/A 493.1411 (b)(3)(ii) clinical laboratory science specialty/subspecialty for which TC is responsible BACHELOR’S : chemical, physical, biological, 2 years laboratory training or experience in the N/A 493.1411 (b)(4)(ii) clinical laboratory science, or medical technology designated specialty/subspecialty for which TC is responsible CLINICAL CONSULTANT Qualifies as a LABORATORY DIRECTOR under N/A N/A 493.1417 (a) 493.1405 (b)(1), (2), or (3)(i) State Licensed Physician N/A N/A 493.1417 (b) MD, DO, DPM TESTING PERSONNEL State Licensed Physician N/A N/A 493.1423 (b)(1) MD, DO OR Ph D, MASTER’S, BACHELOR’S : chemical, physical, biological, clinical laboratory science, or medical technology ASSOCIATE degree: chemical, physical, N/A N/A 493.1423 (b)(2) biological science, or medical laboratory technology HIGH SCHOOL DIPLOMA or equivalent Successfully completed 50 weeks U.S. military medical N/A 493.1423 (b)(3) laboratory procedures training course and held position of Medical Laboratory Specialist (Laboratory Technician) Documentation of appropriate training prior to testing N/A 493.1423 (b)(4)(ii) 4 of 18 CMS Basic EM Tool 07/2006 to 2011 ROs 6/9/CO CLIA Training
09302009 SUMMARY PERSONNEL QUALIFICATIONS – HIGH COMPLEXITY EDUCATION TRAINING/EXPERIENCE BOARD CERTIFIED/ELIGIBLE REGULATION LABORATORY DIRECTOR State Licensed Lab Director (N/A in TX) N/A Certified in Anatomic or Clinical Pathology or both 493.1443 (b)(1)(ii) AND by the American Board of Pathology or the American State Licensed Physician Osteopathic Board of Pathology OR board eligible MD, DO, DPM 1 year laboratory training during medical residency N/A 493.1443 (b)(2)(i) 2 years experience directing or supervising high N/A 493.1443 (b)(2)(ii) complexity testing PhD : chemical, physical, biological, clinical N/A Certified by : 493.1443 (b)(3)(i) laboratory science American Board of Bioanalysis, American Board of Clinical Chemistry, American Board of Forensic Toxicology, American Board of Histocompatibility and Immunogenetics, American Board of Medical Genetics American Board of Medical Laboratory Immunology, American Board of Medical Microbiology, National Registry for Clinical Chemists or other board deemed comparable by HHS N/A Before 02/24/2003 : must have served or be serving as 493.1443 (b)(3)(ii)(B) director of a laboratory performing high complexity testing AND 2 years of laboratory training or experience AND 2 years of laboratory experience directing or supervising high complexity testing. On or before 02/28/92: be serving as a N/A N/A 493.1443 (b)(4) LABORATORY DIRECTOR and must have previously qualified or could have qualified as a LD under 493.1415 published 03/14/90. On or before 02/28/92 be qualified under state N/A N/A 493.1443 (b)(5) law to direct a laboratory in the state in which the lab is located DDS (Licensed) N/A For subspecialty of Oral Pathology only: be 493.1443 (b)(6) certified by the American Board of Oral Pathology, American Board of Pathology, or American Osteopathic Board of Pathology. 5 of 18 CMS Basic EM Tool 07/2006 to 2011 ROs 6/9/CO CLIA Training
Recommend
More recommend