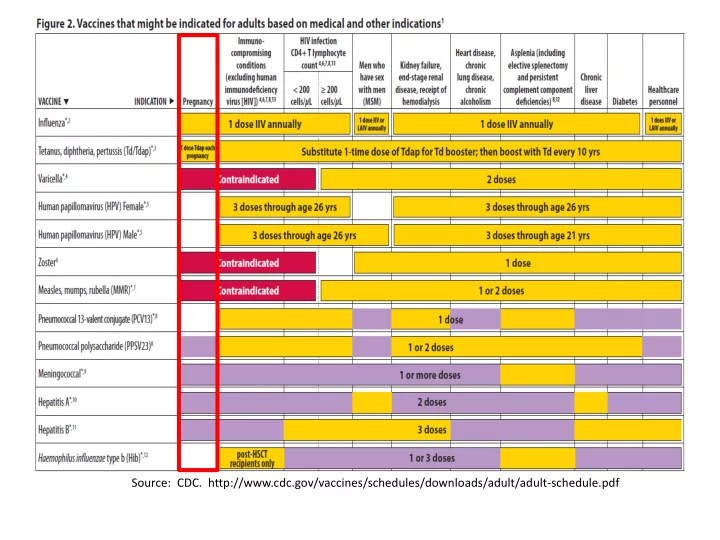

Source: CDC. http://www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule.pdf
Protecting children (& mom) with vaccines begins before conception PRECONCEPTION
Preconception Vaccines • Consider any “pre-conception” visit as an excellent opportunity to update immunization history and catch-up on any missed doses • Especially Think: MMR and Varicella – Vaccines that are contra-indicated during pregnancy – ie. LIVE viral vaccines – Also consider protecting families who are pursuing international adoption!
Congenital Varicella Syndrome • varicella infection in the first 20 weeks' gestation – incidence is estimated to be about 2% • characteristic symptoms • skin lesions in dermatomal distribution (76%) • neurologic defects (60%) • eye diseases (51%) • skeletal anomalies (49%) • 30% of infants die in the first months of life Source: Sauerbrei A, Wutzler P. The congenital varicella syndrome. J Perinatol. 2000 20:548-54.
Congenital Rubella Syndrome • CRS results from rubella virus infection during pregnancy – risk is highest during the first 12 weeks of gestation – decreases after the 12th week of gestation – defects rare after the 20th week of gestation • Serious consequences – Miscarriages and Stillbirths – constellation of severe birth defects • cataracts, congenital heart disease, hearing impairment, and developmental delay • hearing impairment is the most common single defect Source: CDC. http://www.cdc.gov/vaccines/pubs/surv-manual/chpt15-crs.html Martínez-Quintana E, Castillo-Solórzano C, Torner N, Rodríguez-González F. Congenital rubella syndrome: a matter of concern. Rev Panam Salud Publica. 2015;37(3):179–86.
Benefit of Maternal Vaccination • Randomized controlled study of 340 mothers (3 rd Trimester) • Infants of vaccinated mothers had less influenza – 6 cases vs. 16 cases – Vaccine effectiveness of 63% (95% CI, 5 to 85) • Among mothers, reduction of 36% in respiratory illness with fever (95% CI, 4 to 57) Zaman K, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med . 2008;359:1555-1564. JAMA Pediatrics 2016: doi:10.1001/jamapediatrics.2016.0921
Influenza and Pregnancy • UK study of 221 maternity hospitals – 256 pregnant women admitted with confirmed H1N1 – 1220 pregnant women for comparison Image: CDC • Outcomes James Gathany – Perinatal mortality ↑ in infants of infected women 39/1000 vs. 7/1000 (P < 0.001) • – Increase in the rate of stillbirth 27/1000 vs. 6/1000 (P = 0.001) • – Increase in premature birth • adjusted OR = 4.0, 95% CI 2.7 to 5.9 Perinatal outcomes after maternal 2009/H1N1 infection: national cohort study. BMJ. 201; 342:d3214
Safety and Efficacy of Tdap • VAERS Study – No new unexpected vaccine safety concerns – Limited number of pregnancy reports with repeat doses – CDC will continue to monitor • VSD study – Increased risk for chorioamnionitis • RR = 1.11 (1.03-1.21) 5.5% of unvaccinated; 5.6% of vaccinated – Decreased risk of pre-term labor • RR = 0.83 (0.77-0.90) 7.8% of unvaccinated; 5.3% of vaccinated • Vaccine Effectiveness - UK evaluation – Vaccine effectiveness estimated to be 91% (95% CI 84 to 95) Moro P. Safety of Tdap vaccine during pregnancy: enhanced surveillance in VAERS. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2014-02/02-Tdap-Moro.pdf Kharbanda EO et al. Receipt of pertussis vaccine during pregnancy across 7 Vaccine Safety Datalink Sites. Prev Med. 2014. doi: 10.1016/j.ypmed.2014.05.025. Amirthalingam G et al. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet. 2014; doi: 10.1016/S0140-6736(14)60686-3.
Recommend
More recommend