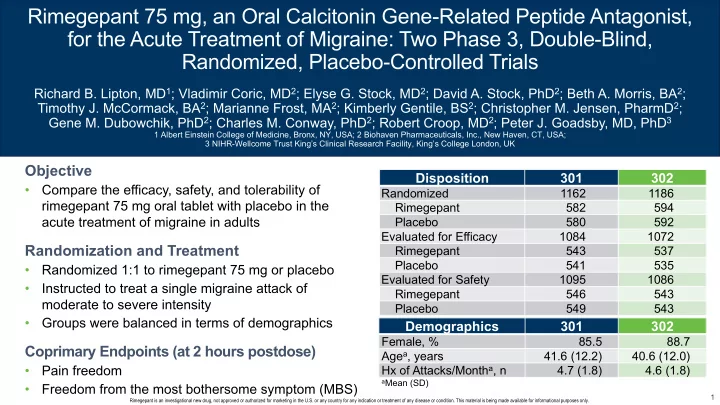

Rimegepant 75 mg, an Oral Calcitonin Gene-Related Peptide Antagonist, for the Acute Treatment of Migraine: Two Phase 3, Double-Blind, Randomized, Placebo-Controlled Trials Richard B. Lipton, MD 1 ; Vladimir Coric, MD 2 ; Elyse G. Stock, MD 2 ; David A. Stock, PhD 2 ; Beth A. Morris, BA 2 ; Timothy J. McCormack, BA 2 ; Marianne Frost, MA 2 ; Kimberly Gentile, BS 2 ; Christopher M. Jensen, PharmD 2 ; Gene M. Dubowchik, PhD 2 ; Charles M. Conway, PhD 2 ; Robert Croop, MD 2 ; Peter J. Goadsby, MD, PhD 3 1 Albert Einstein College of Medicine, Bronx, NY, USA; 2 Biohaven Pharmaceuticals, Inc., New Haven, CT, USA; 3 NIHR-Wellcome Trust King’s Clinical Research Facility, King’s College London, UK Objective Disposition Disposition 301 301 302 • Compare the efficacy, safety, and tolerability of Randomized Randomized 1162 1162 1186 rimegepant 75 mg oral tablet with placebo in the Rimegepant Rimegepant 582 582 594 acute treatment of migraine in adults Placebo Placebo 580 580 592 Evaluated for Efficacy Evaluated for Efficacy 1084 1084 1072 Randomization and Treatment Rimegepant Rimegepant 543 543 537 Placebo Placebo 541 541 535 • Randomized 1:1 to rimegepant 75 mg or placebo Evaluated for Safety Evaluated for Safety 1095 1095 1086 • Instructed to treat a single migraine attack of Rimegepant Rimegepant 546 546 543 moderate to severe intensity Placebo Placebo 549 549 543 • Groups were balanced in terms of demographics Demographics Demographics 301 301 302 Female, % Female, % 85.5 85.5 88.7 Coprimary Endpoints (at 2 hours postdose) Age a , years Age a , years 41.6 (12.2) 41.6 (12.2) 40.6 (12.0) • Pain freedom Hx of Attacks/Month a , n Hx of Attacks/Month a , n 4.7 (1.8) 4.7 (1.8) 4.6 (1.8) a Mean (SD) • Freedom from the most bothersome symptom (MBS) 1 Rimegepant is an investigational new drug, not approved or authorized for marketing in the U.S. or any country for any indication or treatment of any disease or condition. This material is being made available for informational purposes only.
Met Coprimary and Key Secondary Endpoints in Both Studies Sustained Efficacy through 48 Hours on Multiple Measures After a Single Dose of Rimegepant Study 301 302 Rimegepant Placebo Rimegepant Placebo Endpoints P-value P-value N=543, n (%) N=541, n (%) N=537, n (%) N=535, n (%) C OPRIMARY ( AT 2 HOURS POSTDOSE ) Pain freedom 104 (19.2) 77 (14.2) 0.0298 105 (19.6) 64 (12.0) 0.0006 Freedom from the MBS 199 (36.6) 150 (27.7) 0.0016 202 (37.6) 135 (25.2) <0.0001 S ECONDARY a Photophobia-free at 2 hours 164 (34.9) 120 (24.8) 0.0005 b <0.0001 f 183 (37.4) 106 (22.3) Phonophobia-free at 2 hours 133 (38.6) 113 (30.9) 0.0299 c 0.0039 g 133 (36.7) 100 (26.8) Pain relief at 2 hours 304 (56.0) 247 (45.7) 0.0006 312 (58.1) 229 (42.8) <0.0001 Nausea-free at 2 hours 149 (46.9) 134 (41.6) 0.1815 d 171 (48.1) 145 (43.3) 0.2084 h Rescue medication within 24 hours 111 (20.4) 172 (31.8) <0.0001 113 (21.0) 198 (37.0) <0.0001 Sustained pain-free, 2-24 hours 76 (14.0) 44 (8.1) 0.0020 66 (12.3) 38 (7.1) 0.0040 Sustained pain relief, 2-24 hours 211 (38.9) 151 (27.9) 0.0001 229 (42.6) 142 (26.5) <0.0001 Sustained pain-free, 2-48 hours 63 (11.6) 39 (7.2) 0.0130 53 (9.9) 32 (6.0) 0.0181 Sustained pain relief, 2-48 hours 183 (33.7) 129 (23.9) 0.0003 195 (36.3) 121 (22.6) <0.0001 Pain relapse from 2 to 48 hours 41 (40.1) 38 (50.0) 0.1798 e 52 (49.6) 32 (50.0) 0.9648 i Ability to function normally at 2 hours 181 (33.3) 118 (21.8) <0.0001 0.0007 175 (32.6) 125 (23.4) S ELECTED E XPLORATORY Nausea-free at 3 hours 171 (53.8) 139 (43.2) 0.0069 d 209 (58.9) 167 (49.7) 0.0156 h Sustained ability to function normally, 2-48 hours 110 (20.3) 69 (12.8) 0.0008 105 (19.6) 67 (12.5) 0.0016 Sustained freedom from the MBS, 2-48 hours 117 (21.6) 71 (13.1) 0.0002 112 (20.9) 65 (12.2) 0.0001 a Endpoints were tested hierarchically in the order shown at P =0.05; b-i The following numbers indicate the number of subjects in the denominator for each percentage: b Rimegepant n=470, Placebo n=483; c Rimegepant n=345, Placebo n=366; d Rimegepant n=318, Placebo n=322; e Rimegepant n=104, Placebo n=77; f Rimegepant n=489, Placebo n=477; g Rimegepant n=362, Placebo n=374; h Rimegepant n=355, Placebo n=336; i Rimegepant n=105, Placebo n=64 2
Rimegepant Safety and Tolerability Profile Similar to Placebo Study Study 301 301 302 Rimegepant Rimegepant Placebo Placebo Rimegepant Placebo A DVERSE E VENTS A DVERSE E VENTS N=546, n (%) N=546, n (%) N=549, n (%) N=549, n (%) N= 543, n (%) N=543, n (%) Subjects with AEs Subjects with AEs 69 (12.6) 69 (12.6) 59 (10.7) 59 (10.7) 93 (17.1) 77 (14.2) Most common AEs with rimegepant Most common AEs with rimegepant Nausea Nausea 5 (0.9) a 5 (0.9) 6 (1.1) 6 (1.1) 10 (1.8) b 6 (1.1) Dizziness (301) / Urinary tract infection (302) 4 (0.7) 2 (0.4) 8 (1.5) b 6 (1.1) Dizziness (301) / Urinary tract infection (302) 4 (0.7) a 2 (0.4) AEs related to treatment AEs related to treatment 3 (0.5) a 3 (0.5) 1 (0.2) 1 (0.2) 10 (1.8) b 3 (0.6) Serious AEs 2 (0.4) a Serious AEs 2 (0.4) a 1 (0.2) 1 (0.2) 1 (0.2) b 2 (0.4) AEs leading to discontinuation AEs leading to discontinuation 0 0 0 0 0 0 0 0 0 Rimegepant Similar to Placebo on Liver Function Tests (Studies 301 and 302 – Combined) Rimegepant n=1089, n (%) Placebo n=1092, n (%) Serum AST or ALT > ULN 24 (2.2) 32 (2.9) Serum AST or ALT > 3x ULN 1 (0.1) 1 (0.1) Serum AST or ALT > 5x ULN 0 0 Bilirubin > 2x ULN 0 0 AE, adverse event; AST, aspartate aminotransferase; ALT, alanine transaminase; ULN, upper limit of normal a Neither subject had been dosed before the onset of the SAEs; b Back pain, unrelated to treatment Conclusions • Two identical RCTs show that in comparison with placebo, rimegepant delivers significant benefits on the coprimary endpoints — 2-hour pain freedom and freedom from the MBS • Safety and tolerability profiles were comparable to placebo 3
Recommend
More recommend