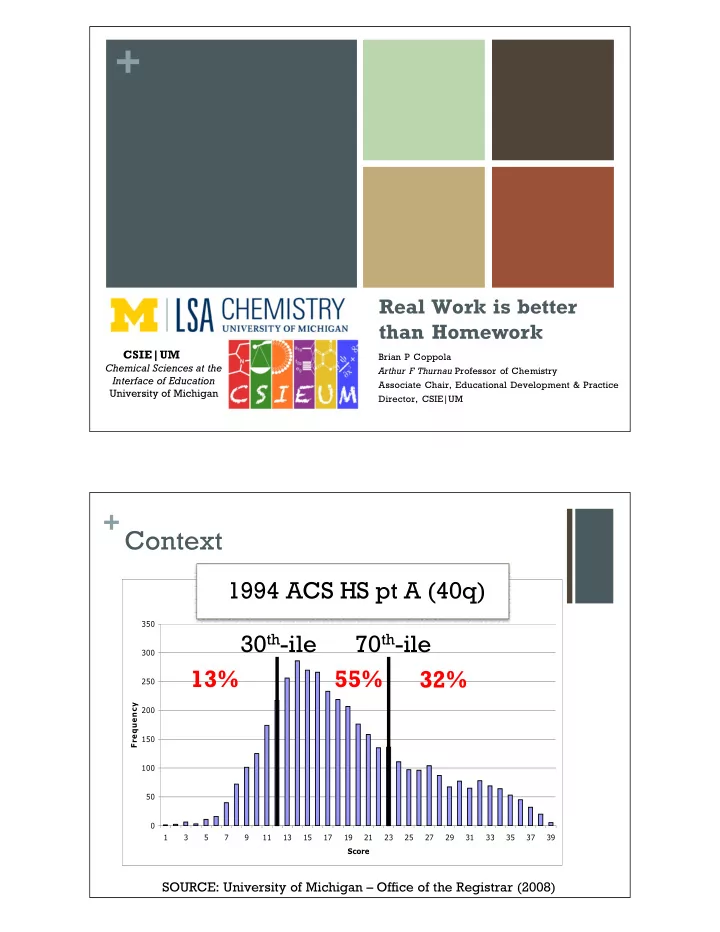

+ Real Work is better than Homework CSIE|UM Brian P Coppola Chemical Sciences at the Arthur F Thurnau Professor of Chemistry Interface of Education Associate Chair, Educational Development & Practice University of Michigan Director, CSIE|UM + Context 1994 ACS HS pt A (40q) Summer 2002 Placement Exam Results 350 30 th -ile 70 th -ile 300 13% 55% 32% 250 Frequency 200 150 100 50 0 1 3 5 7 9 11 13 15 17 19 21 23 25 27 29 31 33 35 37 39 Score SOURCE: University of Michigan – Office of the Registrar (2008)
+ 1989-2015 Earns Gen Chem & Lab Credit 1400 35% AP Logistics: “Letter of Tested Exemption” > 70 th -ile strong alignment in 55% 1 st Yr scope & sequence Organic I Organic II 4 faculty instructors Intro Lab Org Lab 10-12 GSIs 80-90 peer led groups Organic First at the University of Michigan Ege, Coppola, Lawton JCE 1997 , 74 74-83. Coppola, Ege, Lawton JCE 1997 , 74 84-94. + 1989-2015 Earns Gen Chem & Lab Credit (1) atoms are 1400 conserved 35% AP “Letter of Tested (2) reactions are Exemption” > 70 th -ile 55% 1 st Yr not explosions (3) main group is Organic I Organic II well behaved Intro Lab Org Lab The discipline liberates! Coppola & Krajcik JRST 2013 , 50 (6), 627-638. Coppola, Chem. Educator 1996 , 1 (5)
+ 1989-2015 Earns Gen Chem & Lab Credit 1700 1400 65% Eng 35% AP “Letter of Tested 30-70 th -ile Exemption” > 70 th -ile 55% 1 st Yr 95% 1 st ~250 Yr majors Gen Prin Organic I Organic II per year ~350 in 6 Gen Lab Intro Lab Org Lab degree programs intro p-chem (bio)inorg I (bio)analytical analyt lab synth/char phys method + The hidden curriculum Complete the following reaction sequences as required. (a) ACS Med Chem Lett 2014 5 1230 (modified) C 13 H 16 N 2 O 4 Br O NH 2 NH 2 OCH 3 OH Br O H 3 C N H 3 C N K 2 CO 3 (weak base) CH 3 CH 3 50 ˚ C O O 6 hint: the two substitution reactions take place in a specific 8 and predictable order, to produce a single product (b) ACS Med Chem Lett 2014 5 462 1) (H 3 C) 3 C CH 3 BrMg O 1) SOCl 2 low temp OCH 3 O N N 2) H OCH 3 O 2) H 3 O CH 3 N workup CH 3 6 with (CH 3 CH 2 ) 3 N 6 Coppola, B. P. In, Siebert, E. D.; McIntosh, W. J., Eds. College Pathways to the Science Education Standards Arlington, VA: NSTA Press, 2001, 84-86.
+ Real Work Coppola, B. P. “Do Real Work, Not Homework” In, Design Garcia-Martinez, J.; Serrano-Torregrosa, E., Eds. Chemistry Education: Best Practices, Opportunities & Trends Weinheim, Ger.: Wiley-VCH, 2015. Principles • balance of convergent & divergent assignments • balance of teamwork & individual work • use authentic texts (literature) & evidence • peer presentation, review, and critique • students use the instructional technologies • as important to the class as the teacher’s work SUPPLEMENTAL INSTRUCTION + “STUDIO” FOR SCIENCE-MOTIVATED Structured Study Groups Scale: 160 students 2 added hours/week 8 peer-led groups ~20 Varma-Nelson, P.; Coppola, B. P. “Team Learning.” In, Pienta, N.; Cooper, M. M.; Greenbowe, T.; “Chemist’s Guide to Effective Teaching” Saddle River, NJ: Pearson, 2005; 155-169.
+ WEEK #1 sample of student work Divergent Task: find molecule C 13-15 H y Het 1-3 give the citation invent 5 rational isomers rank your invented molecules by melting point by boiling point by dipole moment by water solubility explain ranking (write out) 160 DIFFERENT REPLIES goals: explain & defend In the Group Session: peer review & discussion a chance to change/correct + WEEK #3 Create a quiz/exam problem from a literature source appropriate for the class. The bond indicated by the arrow in compound B has a significantly higher barrier to rotation than the corresponding bond in compound A . Provide a drawing for compound B that best explains this large difference in the ability to rotate that bond. compound A compound B OCH 3 OH N(CH 3 ) 2 N(CH 3 ) 2 N N N N N N H 3 C N(CH 3 ) 2 H 3 C N(CH 3 ) 2 JOC 2012 77 5914–5921
+ Month-long projects: weekly milestones, presentations, reviews Name _______________________ Page 4 IV. (40 points) The following transformation was recently reported ( Org. Lett. 2000 , 2 , 3893). Peer-to-Peer Explanations CH 3 H 3 C H CH 3 I 2 , H 2 O H C OH C C C C C H CH 3 I H Compound K Compound L (a) What is the IUPAC name for Compound K ? 7 (b) What is the hybridization H CH 3 2 2 at each of the indicated carbon atoms? C C C H CH 3 2 2 (c) Draw a 3-dimensional orbital picture for Compound K using lines, dashes and wedges to indicate the sigma bonds, and overlapped p-orbitals for the pi bonds (you may leave the methyl groups indicated as "CH 3 " in your picture. 10 (d) Provide a complete, step-wise mechanism for the formation of Compound L from Compound K . H CH 3 C C C H CH 3 CH 3 H 3 C H C OH C C I H 15 + Another example of student-generated instructional materials E-homework: not used in UM Organic Goal: 100 great skill-based problems in • promotes authoritative answers each of 10 areas with a merciless tutor to • replaces peer interaction bridge text/test gap? A. In 1912, Carl Mannich, a Professor of Pharmaceutical Chemistry at the University of Göttingen, published a paper on a reaction that would come to bear his name: The Mannich Reaction ( Archiv der Pharmazie 1912 , 250 , 647). In the following problem, (a) provide the structure of the intermediate ( A ) that results from the curved arrows shown. Then, (b) using your intermediate, provide the arrows that are needed when intermediate A reacts with acetone enolate to give the observed products. draw the structure of A and the arrows for its reaction Cl with acetone enolate Li O O Li H CH 3 O CH 3 H N O N O O CH 3 O H 3 C Cl H 3 C O acetone O O enolate CH 3 intermediate A 6
+ Another example of student-generated instructional materials E-homework: not used in UM Organic • promotes authoritative answers • replaces peer interaction Goal: 100 great skill-based problems in each of 10 areas with a merciless tutor to bridge text/test gap? Fall: train 170 students to author 200 probs. Spring: select 31 to generate 2 prob./week Summer: test 750 problems/10 skill areas Fall: implement with 1500 students new: edit Org 1 on feedback new: generate Org 2 Baseline item analysis to monitor + Another example of student-generated instructional materials 120 students (second term) 5 sections of ~25… to teach teams of 2-3 get a step • present mechanism • animate mechanism • correlate spectral data • annotate experimental • answer leading questions Create multimedia text • final exam on student text
+ Laboratory courses are a never-ending challenge. SKILLS versus INQUIRY “Who has the same solid material as you do?” Techniques for gathering information: melting point, solubility, tlc, IR Coppola, Lawton 1995 , 72 , 1120-1122 + Laboratory courses are a never-ending challenge. SKILLS versus INQUIRY “Who has the same solid material as you do?” “Who has the same… … liquid? … acid concentration? … numerical series? … dynasty artifact? … enzyme activity? … inhibitor concentration? Coppola, Lawton 1995 , 72 , 1120-1122
+ Laboratory courses are a never-ending challenge. SKILLS versus INQUIRY Week 1: Here are 25 substances, create and separate a binary mixture (600 combinations). Week 2: refine your procedure, purify your compounds. Write up a procedure. Make a couple of samples. Week 3: exchange samples, test others’ procedures. + Laboratory courses are a never-ending challenge. RESEARCH-DRIVEN Week 1: reproduce a literature result (hand out the paper, buy the substrates) Week 2: test some unreported substrates, write up results Week 3: exchange samples, are the results reproducible? Next year: don’t do the same thing
+ Laboratory courses are a never-ending challenge. RESEARCH-DRIVEN Is there a simple procedure being carried out that has not been optimized for yield? 35% + Laboratory courses are a never-ending challenge. RESEARCH-DRIVEN temperature profile team base concentration profile team co-solvent profile team stoichiometry profile team Week 1: replicate literature result Week 2: conduct study Next year: build on results
+ The Interdisciplinary Challenge Core Core Expertise Expertise MS in Postsecondary Combined PhD in Stand-alone PhD in Education for future Chemistry & Education Chemistry Education faculty PhDs Prof. Mark M Banaszak-Holl Drug Transport Agents “research group” on drug structure of the functionalized agents transport based on gathering mechanism of cell incorporation together & organizing the mechanism of drug release desired set of core expertise ultrastructural aspects of cell apoptosis Medical Nanotechnology Macromolecular Physics Science & Engineering + The Interdisciplinary Challenge Core Core Expertise Expertise The historical development of understanding the alcohol dehydrogenase mechanism Week 1: Enzymatic transfer of hydrogen ( J Biol Chem 1953 , 202 , 687) Week 2: Substituent & isotope effects in yeast ADH reaction ( J Biol Chem 1972 , 247 , 7977) Week 3: X-ray structure of active site & mechanism for substrate specificity ( J Biol Chem 1997 , 272 , 18558) Week 4: ADH activity & blood alcohol in women ( NE J Med 1990 , 332 , 95)
Recommend
More recommend