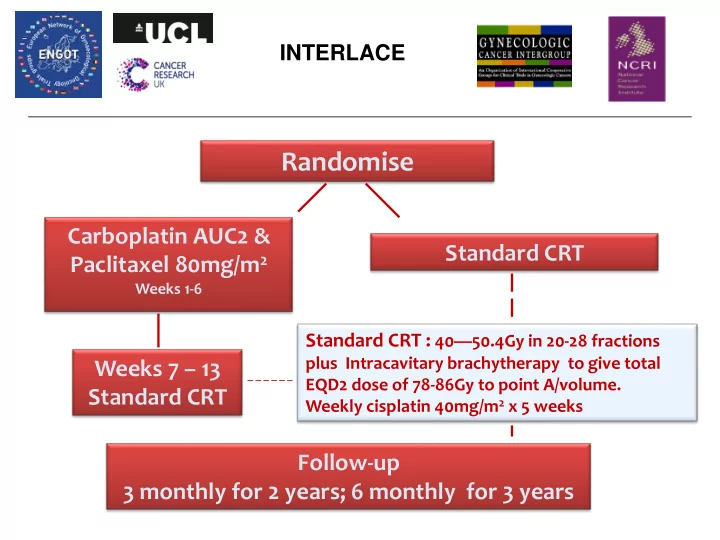

INTERLACE Randomise Carboplatin AUC2 & Standard CRT Paclitaxel 80mg/m 2 Weeks 1-6 Standard CRT : 40 — 50.4Gy in 20-28 fractions plus Intracavitary brachytherapy to give total Weeks 7 – 13 EQD2 dose of 78-86Gy to point A/volume. Standard CRT Weekly cisplatin 40mg/m 2 x 5 weeks Follow-up 3 monthly for 2 years; 6 monthly for 3 years
INTERLACE Eligibility criteria summary • All patients suitable for CRT, FIGO IB1 with +ve nodes-IVA unless: - Nodes above aortic bifurcation - Disease involves lower third of vagina (FIGO IIIA) IMRT permitted Recently received ethical approval for redesign of PIS and inclusion of a short series of patient accessible video links (via PIS) explaining the trial and featuring INTERLACE patient interviews.
INTERLACE INTERLACE Trial - Total Monthly Accrual 14 12 Number of Patients/Sites 10 8 6 4 2 0 Actual Monthy Accrual
INTERLACE Current status Target recruitment – 770 Accrual to date (UK and Mexico) – 172 Number of sites open - 30 • GICOM (Mexico) – 21 patients recruited since opening in Feb 2016 • MaNGO (Italy) – 5 sites in setup
INTERLACE Contacts: Chief Investigator – Dr Mary McCormack mary.mccormack@uclh.nhs.uk RTQA – Yatman Tsang yatmantsang@nhs.net General Enquiries – ctc.interlace@ucl.ac.uk
Recommend
More recommend