
Natural Backgrounds 1
U 238 Decay Chain U 234 U 238 92 92 4.5e9 245,500 Years Years Uranium β ⁻ Pa 234 α α 91 27 Days Protactinium β ⁻ Th Th 234 230 90 90 75,380 27 Days Years Thorium α Actinium Ra 226 88 1602 Years Radium α Francium Rn 222 86 3.8 Days Radon At 218 α 85 1.5 Seconds Astatine Po Po 218 214 Po 210 α 84 84 84 0.1643 3.1 Minutes 138 Days Seconds Polonium β ⁻ β ⁻ Bi Bi 214 210 α α α 83 83 20 Minutes 5 Days Bismuth β ⁻ β ⁻ Pb 214 Pb Pb 210 206 α α 82 82 82 26.8 22.3 Years Stable Minutes Lead β ⁻ β ⁻ Tl 210 Tl 206 α Actinides 81 81 Alkali Metals 1.3 Minutes 4.2 Minutes Thallium Alkaline Earth Metals Halogens Metalloids Hg 206 Noble Gases 80 Poor Metals 8.1 Minutes Mercury Transition Metals
TH 232 Decay Chain Th 232 Th 228 90 90 1.41e+10 1.9 Years Years Thorium β ⁻ Ac 228 α α α 89 6.1 Minutes Actinium β ⁻ Ra Ra 228 224 α 88 88 5.7 Years 3.6 Days Radium α Francium Rn 220 86 55 Seconds Radon α Astatine Po Po 216 212 84 84 0.14 3e-07 Seconds Seconds Polonium β ⁻ Bi 212 α α 83 61 Minutes Bismuth β ⁻ Pb Pb 212 208 α Actinides 82 82 Alkali Metals 10.6 Stable Minutes Alkaline Earth Metals Lead Halogens β ⁻ Metalloids Tl 208 Noble Gases 81 Poor Metals 3.1 Minutes Transition Metals Thallium
Radon 4
Radon Hot Spots in US 5
Radon Hot Spots in NJ 6
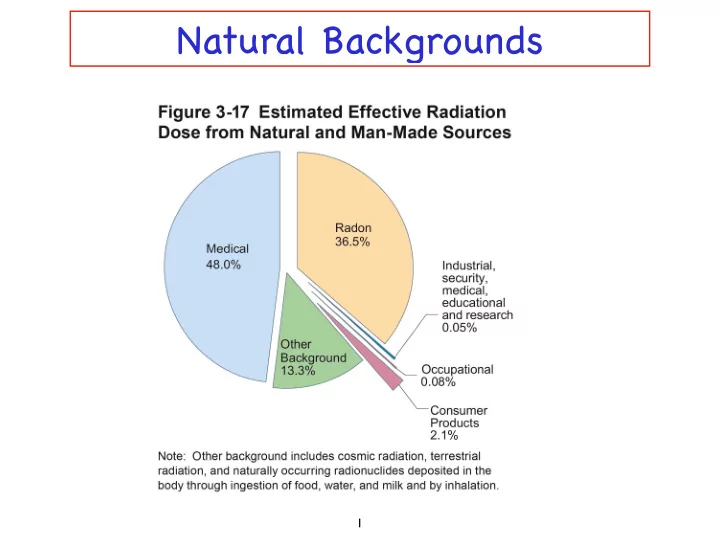
Radon Heavy noble gas 200 mrem per year Largest source of radiation exposure One in a hundred chance of inducing cancer over a person’ s lifetime Main cause of lung cancer among nonsmokers By product of uranium decay 19 7
Natural Radioactivity Average dose is 300 to 400 mrem (0.3 to 0.4 rem) Dose from living next to a nuclear power plant is 30,000 times less 8
Main Takeaway Points • Microwave radiation does not cause cancer • Risk from medical x-ray is small - full body CT scan about 3 times average annual background dose - benefits far out weigh risks • Radiation is everywhere - cosmic rays - Uranium and Thorium in soil - Potassium in your body • Average annual back ground dose about 300 mrem - About 1% chance of inducing cancer over a lifetime • Largest exposure for nearly everyone is Radon - Radioactive gas that can seep into basements from the ground - Radon levels in northwestern New Jersey higher than average - If you live there, have your house radon level tested - If above threshold level, undertake remediation • What are the benefits and cons of nuclear power 9
Damage to DNA 10
Damage to DNA 11
Effect on Living Tissue Radiation can ionize (remove electrons from) atoms This breaks molecular bonds The DNA molecules that contain our genetic code are particular susceptible to radiation damage. DNA is a very fragile molecule. Its bonds can easily be broken. Harmful effects of radiation Cancer If the gene on the DNA is mutated it can lead to the growth of caner cells Radiation sickness / death A large enough radiation exposure can cause large amount of cell death leading to sickness and, if enough, death 12
Doses Whole body doses Below 100 rem no short term illness 100 to 200 rem short term illness 300 rem 50% chance of death More than 1000 rem survival unlikely 13
Cancer Dose Whole body dose needed to for 2500 rem near certainty of inducing cancer This is greater than the lethal dose! Even small doses have some probability of inducing cancer and it is likely cumulative over your lifetime Assume probability risk of cancer linearly proportional to dose Assume no threshold 25 rem exposure would mean a 1% risk of cancer If 100 people are exposed to 25 rem, we expect 1 to get cancer Even with no radiation exposure 20% of people will get cancer 14
Linear Hypothesis 15
Summary of Cancer Risks Lifetime total natural exposure 1 in 100 (1.0%) Lifetime radon exposure 1 in 150 (0.7%) One medical x-ray 1 in 60,000 Lifetime self exposure 1 in 100,000 Living 50 years next to 1 in 500,000 nuclear power plant 1 in 5 (20%) Non radiation causes Radiation from routine operation of a nuclear power plant is not a health issue 16
Atomic Nucleus The nucleus at the center of each atom is made up of protons and neutrons Protons and neutrons have approximately equal masses and each is about 2000 times more massive than an electron. Nearly all of the mass of an atom is in the nucleus The number of protons in the nucleus is equal to the number of electrons in the electron cloud The atomic number, Z, is the number of protons The atomic weight, A, is the number of protons plus neutrons The nucleus is 100,000 times smaller than the atom If an atom were the size of the Rutgers football stadium the nucleus would be the size of a mosquito 17
Chemical (Atomic) Energy In a chemical reaction electron binding energy is converted into kinetic energy Example the burning of ethane (a hydrocarbon) H H H H + + O C O H C H O O O H H + + H 2 O 6 4 CO 2 7 O 2 2 C 2 H 6 The daughter molecules are more tightly bound together than the parent molecules There is less chemical potential energy in the final state than in the initial. Chemical potential energy is converted to kinetic energy (heat) 18
Nuclear Binding Energy A nuclear reaction is similar + + 3 neutrons 236 U 92 Kr 141 Ba The daughter nuclei are more tightly bound together than the parent nucleus There is less nuclear potential energy in the final state than in the initial. Nuclear potential energy is converted to kinetic energy (heat) Nuclear binding energy is 1 to 10 million time stronger than chemical binding energy 19
Chemical vs. Nuclear Energy Because of the great difference in binding energies, for as given, mass, tremendous amount more energy is released in a nuclear reaction than in a chemical reaction Burning 1 gram of gasoline releases 42 kJ Fissioning 1 gram of Uranium releases 82 GJ In both cases we need a trigger. Ethane won’ t burn on it own and uranium won’ t fission on its own In the case of a chemical reaction the trigger is a spark or heat In the case of a nuclear fission reaction the trigger is the nucleus being struck by an energetic neutron 20
Binding Energy of Nuclei Iron (Fe) is the most tightly bound nucleus Nuclei more massive than Fe can release energy by fissioning to lighter nuclei Nuclei less massive than Fe can release energy by fusing to form heavier nuclei 21
Nuclear Fission In a nuclear fission reaction a heavy parent nuclei fissions (or splits) into two or more lighter daughter nuclei. Several neutrons may be released in the process as well In general the heavier nuclei have a greater tendency to fission 22
235 U Fission If a neutron hits a 235 U nucleus it will form a 236 U nucleus that then fissions into lighter nuclei (fragments) 23
Chain Reaction A chain reaction occurs if the fission process releases one or more neutrons that can then initiate addition fission reactions If a fission releases two neutrons after a few generations there will be many fissions. This all happens very rapidly generation number of fissions 2 1 = 2 1 2 2 = 4 2 2 3 = 8 3 5 2 5 = 32 10 2 10 = 1000 2 20 = 10 6 20 2 40 = 10 12 40 2 80 = 10 24 80 24
Uncontrolled Chain Reaction If the chain reaction runs its course after 84 generations 10 kg of Uranium will have fissioned. Per weight, Uranium releases 30,000,000 times as much energy as TNT. 10 kg of Uranium corresponds to 300 kilotons of TNT We have a bomb In order for this to work two things are necessary. 1) Critical mass. There must be enough Uranium so that the neutrons interact before they escape. 2) Enrichment. There must a large enough fraction of 235 U compared to 238 U since 238 U absorbs the neutrons. Critical mass: 15 kg Enrichment: 90% 25
Controlled Chain Reaction The chain reaction can be controlled by using a material that absorbs neutrons By controlling the amount of absorber, we can arrange so that for every fission there in only one neutron available to trigger another fission. Then the reaction won’ t run away but will run at constant power. We have a nuclear reactor 26
Neutron Moderation Slow neutrons are much more effective in causing fission than fast neutrons Use a moderator that scatters neutrons but doesn’ t absorb them. An example is water After enough scattering the neutrons will be thermal. They will have energy corresponding to the ambient temperature. 27
Nuclear Reactor 28
Light Water Reactor Most reactors are moderated with light (normal) water Two types: pressurizer water (above) or boiling water. They require that the fuel be enriched to 3% to 4% 235 U 29
Fuel Rods Compacted fine powder of enriched Uranium oxide sintered into pellets and placed into long zircalloy tubes Fuel enriched to about 3% 235 U About 100 tons of fuel per year for 1 GW reactor 30
Control Rods Control rods usually contain boron since it is a very good neutron absorber 31
Enrichment Naturally occurring uranium is only about 0.7% 235 U Since the light water moderator also absorbs neutrons need to enrich to about 3% 235 U. Heavy water moderator reactor uses unenriched fuel Enrichment needed for bomb is 20% minimum but efficient bomb requires 90% since don’ t want 238 U to absorb the neutrons 32
Recommend
More recommend