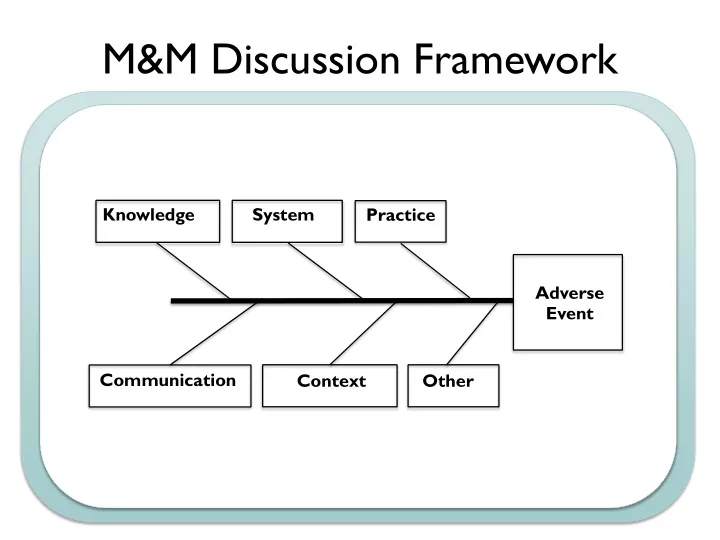

M&M Discussion Framework Knowledge System Practice Adverse Event Communication Context Other
EX: Sepsis Mortality Equipment Process People - EMR does not provide - Time to antibiotics - Staffing in ED SIRS alerts - Enough fluid - PharmD in ED - POC lactate machine administration - Provider knowledge of - Diagnostic testing and - Triage to right level of care sepsis guidelines source control not - Culture not to call for VS available 24/7 changes Materials Management Environment - No incentives for good - Enough blood culture - Sicker patients sepsis performance bottles - ED crowding - No resources for data - The right abx are not - Trauma patients take collection available quickly priority - No system for audit and feedback
QI Project Application Form QI Project Application Form Adapted for UCSF from ABP Project Basics 1. Quality Improvement Project Title. 2. Quality Improvement Project Leader (faculty member, only one needed per project). 3. Briefly comment on the Quality Improvement Project Leader’s experience, highlighting any experience and expertise relevant to quality improvement. 4. Start date of the project. 5. End date of project. 6. Date of Application. 7. Please comment on the involvement of trainees (residents, students, etc.) in this project. Core Project Components Improvement Aims 8. Briefly describe the problem the QI project addresses. What is the nature and severity of the problem? What is the gap between current practice and what is possible? Why did you choose to work on this problem? Please limit response to 300 words or less. 9. What is the specific aim of the QI project? In your response, identify the patient population and measurable, time-specific objectives of the project. Please limit response to 300 words or less.
QI Project Application Form 10. Which institute of Medicine Quality Dimensions are addressed by the project? (Check all that apply). Safety Timeliness Effectiveness Equity Efficiency Patient-Centered Measurement 11. Provide information for each measure used in the project by completing the measures specification template. Outcome measure (describe, include measurement and target/goal): Balancing measure (describe, including measurements if applicable): Process measure (describe, including measurement if applicable): 12. If you used sampling, describe the project’s sampling strategy and why it is appropriate. 13. How often are data reviewed; what is the data collection cycle for the project? (Check one.) Monthly Quarterly Other (specify) 14. Explain data collection process.
QI Project Application Form Interventions 15. Describe the project’s intervention(s) or changes for improvement. Why were they selected? All projects should have at least two cycles of change. 16. What are the two biggest barriers (known or anticipated) to implementing the interventions/changes, and how will you address the barriers? Results, Analysis, and Reporting 17. At what level are data analyzed and reported? (Check all that apply.) Individual physician level Clinic-group level Aggregated across multiple clinic-groups 18. How often are results (performance feedback) provided to participants? (Check one.) Monthly Quarterly Other (specify) 19. How are results tracked by the project leaders and reported to participants (performance feedback)? Run charts and control charts are basic statistical process control charting methods, and the ABP strongly recommends that projects include one or both as the method of tracking results. If your project is not using run or control charts, explain why you are using an alternative format. 20. Explain how the project uses data to guide improvement.
QI Project Application Form 21. What are the results to date for each measure? Provide the most recent chart for each measure, using aggregate data (across all participants). Comment briefly on the results. Select the QI project’s score on the Improvement Progress Scale from the table below. Explain the basis for the score. (Check one.) Improvement Progress Scale 1 1.0 Design Phase Project design complete. 1.5 Baseline All participant teams formed. Baseline measurement begun. 2.0 Local adaptations Teams meeting routinely. Interventions being adapted for local factors by teams. All teams planning first tests of change (PDSAs). 2.5 Tests of change & data Teams have conducted first tests of change (PDSAs). collection All teams reporting data. No improvement in quality measures yet. 3.0 Modest process All teams have conducted multiple tests of change (PDSA cycles). improvements Process measures beginning to show improvement. 3.5 Improvement Process measures continue to improve. Some improvement in at least one outcome measure. 4.0 Significant improvement Evidence of sustained improvement in outcome measures. Halfway toward accomplishing all aims (goals). Plans for spread of improvements are formulated. 4.5 Sustainable improvement Sustained improvement in most outcome measures. 75% of aims (goals) achieved. Spread of improvements has begun. 5.0 Outstanding sustainable All interventions implemented. results All aims achieved. Improvements have spread to new settings. 1 Adapted with permission from the Institute for Healthcare Improvement’s Assessment Scale for Collaboratives at www.ihi.org/IHI/Topics/Improvement/ImprovementMethods/Tools .
QI Project Application Form Physician Participation At UCSF physicians must be involved for a minimum of 6 months, with a minimum of 2 cycles of change. 22. Describe what is expected of each physician participating in the project. What is the physician’s role in implementing the intervention(s); collecting, submitting and analyzing data; participation in team meetings; and modifying the intervention(s) based on the data? Physician Attestation Processes 23. Pediatricians seeking MOC credit must complete the ABP Attestation Form, which is co-signed by the Project Leader or by a “Local Leader”, depending on the project’s structure. This co-signing Leader is responsible for adjudicating any disputes with physicians who wish to claim credit for MOC. Because this process could affect a physician’s certification status, the co-signing Leaders should be physicians who are active participants in the approved projects. Because of the nature of the UCSF projects, Project Leaders will complete the Attestation Form and it will be co-signed by Glenn Rosenbluth as a “Local Leader” 24. Provide the most recent progress report (e.g. a snapshot of the wiki-site). How and how often does the project leadership keep key stakeholders informed of progress and results? 25. Explain how the project is HIPAA compliant. 26. Does the project have IRB approval? (Check one.) We did not seek IRB approval because it is not required. We did seek IRB approval because we hope to disseminate our results.
PHM QI Workshop Case: Neonatal Readmission Summary: Term male infant admitted with fever at 2 days of age and treated for early onset GBS sepsis without complications Re-admitted at 3 weeks of age with seizure and lethargy in the setting of hypoglycemia, hyponatremia, and dehydration. Admission #1: “fever” • HPI: Term M infant born at home without complications & exclusively breastfeeding. On DOL#2-3 he developed decreased feeding frequency over 24hrs (no PO intake x 8hrs); decreased UOP (last wet diaper >18hrs ago). He has been sleepy but arousable. Rectal temp is 38.9C at home. • PMHx: o Prenatal: 29yo G2P1-2 mother; GBS-; other serologies unremarkable o Birth: NSVD @ 39wks GA; midwife-attended home birth; Apgars 9/9; BW 3400gm • SHx: Lives in Ukiah with mother, father, 15mo sibling. Mother had prior successful homebirth + exclusively breastfed sibling. Recent social stressors: father lost job 1mo ago, mother has h/o postpartum depression. • ED Course: Febrile, tachycardic, lethargic infant. Sepsis work-up initiated. Umbilical line placed for IVF and antibiotics; Ampicillin and Gentamicin administered. • Hospital Course: o Sepsis: BCx +GBS @ 12hrs; UCx, CSF Cx negative IV abx x 10 days. o Dehydration / Feeding Difficulty: IVF x 3 days formula supplementation + minimal breastfeeding (mother could not room-in due to young sibling + distance from home). • Discharge: DOL#12. Admission #2: “poor feeding” • HPI: 3-week-old M infant brought to ED for lethargy, dehydration. Infant had difficulty with breastfeeding (diminished supply, poor latch) so family started supplementing with formula (mixed w/ extra water to conserve powdered formula), cow’s milk, goat’s milk, tea, and water. • ED Course: Poorly responsive, tachycardic infant with witnessed seizure in triage. Diagnostic evaluation revealed hyponatremia (Na 122), hypoglycemia (glucose 40), and dehydration. • PICU Course: o Metabolic derangements: hyponatremia and hypoglycemia corrected with IVF. o R/o sepsis: work-up with empiric antibiotics x 48hrs negative. o NAT work-up: negative. o Tox work-up: negative. o Dehydration / Feeding Difficulty: IVF x 2 days Infant able to feed well PO with formula via bottle. • Transfer: Pediatric Hospitalist service on HD#3. What Happened?
What Is Quality Improvement and Why Should Pediatric Hospitalists Care? Arpi Bekmezian, MD Director of Quality and Safety, Division of Pediatric Hospital Medicine Associate Medical Director of Quality and Safety, UCSF Benioff Children’s Hospital Assistant Clinical Professor, Department of Pediatrics Katey Hoffman, MD Clinical Quality Liaison, UCSF-Marin General Hospital Pediatric Care Affiliation Assistant Clinical Professor, Department of Pediatrics ?? Pediatric Hospitalist, Marin General Hospital
Recommend
More recommend