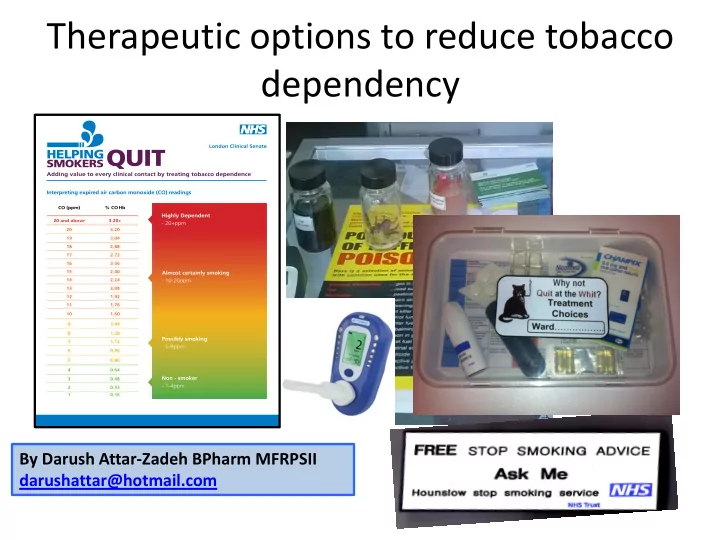

Therapeutic options to reduce tobacco dependency By Darush Attar-Zadeh BPharm MFRPSII darushattar@hotmail.com
Need to have the right resources available at http://www.londonsenate.nhs.uk/wp-content/uploads/2015/04/The-expired-carbon-monoxide-CO-test-guidance-for-health- professionals.pdf
Why is it hard for your patient to stop smoking? • Short half life of nicotine requires smokers to regularly smoke to maintain levels 2 • Reinforcing desired effects of nicotine with each cigarette soon becomes addictive 2 1. Russell MAH, et al. BMJ 1976; 1:1043-1046 2. Nicotine addiction in Britain: A report of the Tobacco Advisory Group of the Royal college of Physcians. London: Royal College of Physicians, 2000
Champix update - ‘EAGLES study (EVALUATING ADVERSE EVENTS IN A GLOBAL SMOKING CESSATION STUDY)
Keep the message simple! Varenicline at the 4 2 receptor Partial agonist Antagonist • Prevents stimulation of the receptor • Binds with high affinity to the 4 2 receptor, only partially stimulating by nicotine dopamine release 1 • This reduces the pleasurable effects • Provides relief from craving and of smoking and potentially the risk of withdrawal symptoms 1-3 full relapse after a temporary lapse 1-4 1 . Coe JW. J Med Chem 2005; 48:3474-3477. 2 . Gonzales D et al. JAMA 2006; 296:47-55. 3 . Jorenby DE et al. JAMA 2006; 296:56-63. 4 . Foulds J. Int J Clin Pract 2006; 60:571-576.
6 8144 smokers 2037 varenicline 4028 non-psych; 4116 psych 2034 bupropion 16 countries, 6 continents 2038 NRT 30 th Nov 2011- 13 th Jan 2015 2035 placebo
Key Inclusion Criteria 1 • Smokers ages 18 to 75, average ≥10 cigarettes/day and have an exhaled carbon monoxide (CO) of >10 ppm at screening • Determined by SCID (Structured Clinical Interview for DSM-IV Disorders) to have either: No current or past psychiatric diagnosis (50% of subjects) OR One or more clinically stable current or past diagnosis of (50% of subjects): Psychotic disorder – schizophrenia, schizoaffective disorder Affective disorder – major depression, bipolar I, bipolar II Anxiety disorder – panic disorder with or without agoraphobia, post- traumatic stress disorder, obsessive-compulsive disorder, social phobia, generalised anxiety disorder Personality disorder – limited to past history of borderline personality disorder Subjects with psychiatric diagnoses must be clinically stable: No acute exacerbation in the preceding 6 months If on treatment, stable drug and dose for ≥3 months 1. Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, Ascher J, Russ C, Krishen A, Evins AE. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet 2016 Apr 22. doi: 10.1016/S0140-6736(16)30272-0 [Epub ahead of print]
Key Exclusion Criteria 1 • All Subjects : • Clinically significant cardiovascular disease or cerebrovascular disease in the past 2 months • Severe COPD • Varenicline, bupropion, or NRT within 30 days of the baseline • Subjects for whom bupropion use is inappropriate (seizure disorder, etc.) • Suicidal risk or display self-injurious behaviour • Unable to comply with study procedures • Subjects who have previously experienced an adverse event that was potentially due to treatment with any of the active drugs in the study, and that is of sufficient concern that further exposure to this medication would be inadvisable • Additional Exclusions for Non-Psychiatric Cohort • Current or past history of any psychiatric disorders • Current or past history of substance abuse • Additional Exclusions for Psychiatric Cohort • Substance abuse not in remission for at least 12 months • Subjects who rate >5 (markedly ill or worse) on the Clinical Global Impression of Severity (CGI-S) 1. Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, Ascher J, Russ C, Krishen A, Evins AE. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet 2016 Apr 22. doi: 10.1016/S0140-6736(16)30272-0 [Epub ahead of print]
Key Endpoints 1 Primary Safety Endpoint: The percentage of subjects reporting at least one of the following Neuropsychiatric (NPS) adverse events (AEs) during treatment and up to 30 days after last dose: Agitation Panic Aggression Paranoia Classified as Delusions Anxiety Moderate Classified as Psychosis Depression Feeling abnormal Hallucinations or Severe Hostility Suicidal ideation Severe Homicidal ideation Suicidal behaviour Mania Completed suicide Main Efficacy Measure: CO-confirmed 4-week continuous abstinence rates (CAR) for Weeks 9-12 Adverse events were rated by trained investigators as mild (no interference with participant’s usual daily functioning), mode rate (some interference with functioning), or severe (substantial interference). Prespecified severity criteria for the primary neuropsychiatric adverse event endpoint required adverse events for the four components expected to be reported more commonly (anxiety, depression, feeling abnormal, or hostility) to be rated as severe. Neuropsychiatric adverse events in the remaining 12 categories (agitation, aggression, delusions, hallucinations, homicidal ideation, mania, panic, paranoia, psychosis, suicidal ideation, behaviour, or completed suicide) met severity criteria when rated as either moderate or severe. 1. Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, Ascher J, Russ C, Krishen A, Evins AE. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet 2016 Apr 22. doi: 10.1016/S0140-6736(16)30272-0 [Epub ahead of print]
Primary Safety Endpoint: Neuropsychiatric AE Composite Endpoint 1 Participants with Events n/N, % Cohort Varenicline Bupropion NRT Patch Placebo 13/990 22/989 25/1006 24/999 Non-Psychiatric 1.3% 2.2% 2.5% 2.4% 67/1026 68/1017 53/1016 50/1015 Psychiatric 6.5% 6.7% 5.2%* 4.9% Moderate to severe neuropsychiatric AEs reported during treatment and ≤30 days after last dose. In a general linear model analysis pre-specified in the protocol, there was a significant treatment-by-cohort interaction. Therefore statistical analyses of the NPS AE endpoint by treatment assignment were conducted for each cohort separately. *One additional participant in the nicotine patch group (psychiatric cohort) who reported moderate suicidal ideation (serious adverse events) was identified after the clinical database was locked; consequently, the participant was not included in the analysis of the primary study endpoint. 1. Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, Ascher J, Russ C, Krishen A, Evins AE. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet 2016 Apr 22. doi: 10.1016/S0140-6736(16)30272-0 [Epub ahead of print]
Secondary Outcome Measure Severe-Only NPS AEs in the Primary Endpoint 1 Varenicline Bupropion NRT Patch Placebo Non-Psychiatric Cohort, N 990 989 1006 999 NPS AE Endpoint, n (%) 13 (1.3%) 22 (2.2%) 25 (2.5%) 24 (2.4%) Severe only 1 (0.1%) 4 (0.4%) 3 (0.3%) 5 (0.5%) Psychiatric Cohort, N 1026 1017 1016 1015 NPS AE Endpoint, n (%) 67 (6.5%) 68 (6.7%) 53 (5.2%)* 50 (4.9%) Severe only 14 (1.4%) 14 (1.4%) 14 (1.4%) 13 (1.3%) Adverse events were rated by trained investigators as mild (no interference with participant’s usual daily functioning), mode rate (some interference with functioning), or severe (substantial interference). Prespecified severity criteria for the primary neuropsychiatric adverse event endpoint required adverse events for the four components expected to be reported more commonly (anxiety, depression, feeling abnormal, or hostility) to be rated as severe. Neuropsychiatric adverse events in the remaining 12 categories (agitation, aggression, delusions, hallucinations, homicidal ideation, mania, panic, paranoia, psychosis, suicidal ideation, behaviour, or completed suicide) met severity criteria when rated as either moderate or severe. *One additional participant in the nicotine patch group (psychiatric cohort) who reported moderate suicidal ideation (serious adverse events) was identified after the clinical database was locked; consequently, the participant was not included in the analysis of the primary study endpoint. 1. Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, Ascher J, Russ C, Krishen A, Evins AE. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet 2016 Apr 22. doi: 10.1016/S0140-6736(16)30272-0 [Epub ahead of print]
Recommend
More recommend