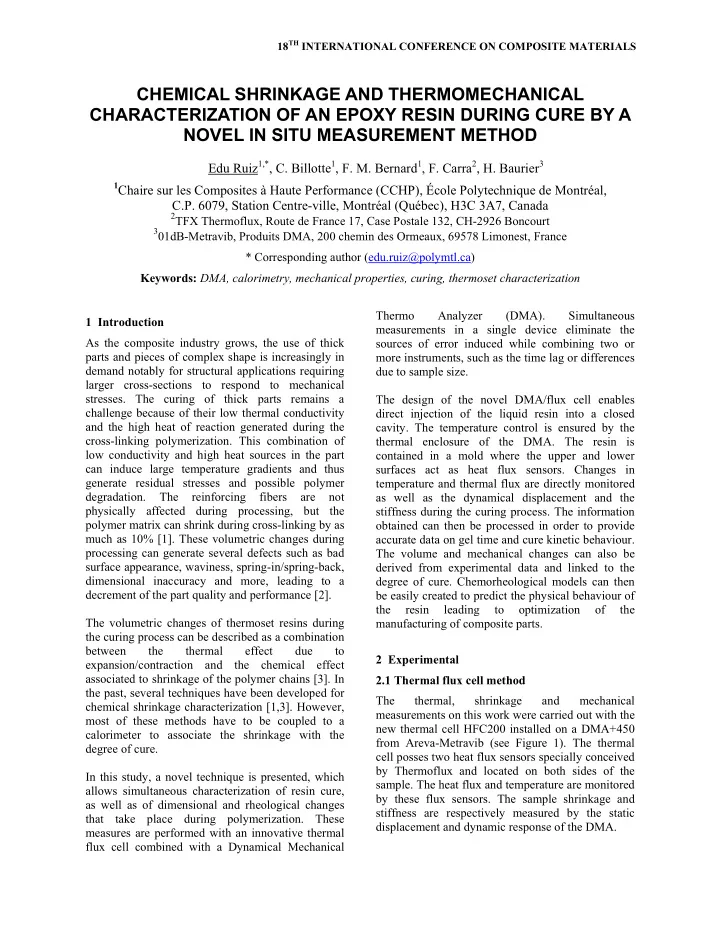

18 TH INTERNATIONAL CONFERENCE ON COMPOSITE MATERIALS CHEMICAL SHRINKAGE AND THERMOMECHANICAL CHARACTERIZATION OF AN EPOXY RESIN DURING CURE BY A NOVEL IN SITU MEASUREMENT METHOD Edu Ruiz 1,* , C. Billotte 1 , F. M. Bernard 1 , F. Carra 2 , H. Baurier 3 1 Chaire sur les Composites à Haute Performance (CCHP), École Polytechnique de Montréal, C.P. 6079, Station Centre-ville, Montréal (Québec), H3C 3A7, Canada 2 TFX Thermoflux, Route de France 17, Case Postale 132, CH-2926 Boncourt 3 01dB-Metravib, Produits DMA, 200 chemin des Ormeaux, 69578 Limonest, France * Corresponding author (edu.ruiz@polymtl.ca) Keywords: DMA, calorimetry, mechanical properties, curing, thermoset characterization Thermo Analyzer (DMA). Simultaneous 1 Introduction measurements in a single device eliminate the As the composite industry grows, the use of thick sources of error induced while combining two or parts and pieces of complex shape is increasingly in more instruments, such as the time lag or differences demand notably for structural applications requiring due to sample size. larger cross-sections to respond to mechanical stresses. The curing of thick parts remains a The design of the novel DMA/flux cell enables challenge because of their low thermal conductivity direct injection of the liquid resin into a closed and the high heat of reaction generated during the cavity. The temperature control is ensured by the cross-linking polymerization. This combination of thermal enclosure of the DMA. The resin is low conductivity and high heat sources in the part contained in a mold where the upper and lower can induce large temperature gradients and thus surfaces act as heat flux sensors. Changes in generate residual stresses and possible polymer temperature and thermal flux are directly monitored degradation. The reinforcing fibers are not as well as the dynamical displacement and the physically affected during processing, but the stiffness during the curing process. The information polymer matrix can shrink during cross-linking by as obtained can then be processed in order to provide much as 10% [1]. These volumetric changes during accurate data on gel time and cure kinetic behaviour. processing can generate several defects such as bad The volume and mechanical changes can also be surface appearance, waviness, spring-in/spring-back, derived from experimental data and linked to the dimensional inaccuracy and more, leading to a degree of cure. Chemorheological models can then decrement of the part quality and performance [2]. be easily created to predict the physical behaviour of the resin leading to optimization of the The volumetric changes of thermoset resins during manufacturing of composite parts. the curing process can be described as a combination between the thermal effect due to 2 Experimental expansion/contraction and the chemical effect associated to shrinkage of the polymer chains [3]. In 2.1 Thermal flux cell method the past, several techniques have been developed for The thermal, shrinkage and mechanical chemical shrinkage characterization [1,3]. However, measurements on this work were carried out with the most of these methods have to be coupled to a new thermal cell HFC200 installed on a DMA+450 calorimeter to associate the shrinkage with the from Areva-Metravib (see Figure 1). The thermal degree of cure. cell posses two heat flux sensors specially conceived by Thermoflux and located on both sides of the In this study, a novel technique is presented, which sample. The heat flux and temperature are monitored allows simultaneous characterization of resin cure, by these flux sensors. The sample shrinkage and as well as of dimensional and rheological changes stiffness are respectively measured by the static that take place during polymerization. These displacement and dynamic response of the DMA. measures are performed with an innovative thermal flux cell combined with a Dynamical Mechanical
2.2 Material and experimental set up differences at the beginning and at the end of polymerization in addition to the time lag mentioned The resin system used in this work is typical Di- previously. Also instabilities may occur because of Glycidyl Ether of Bisphenol A ( DGEBA) epoxy the temperature of the thermal enclosure. anhydride cured. Before resin injection, the temperature is stabilized in the thermal enclosure at The upper plate of the DMA cell oscillates at 120°C. Resin is then injected into the sample holder. amplitude of 20 microns and frequency of 10 Hz. The DMA is then actioned applying a vibrating The instrument applies a controlled force to induce force to the sample at low displacement. such oscillation to the resin sample. As the liquid resin undergoes polymerization, the dynamic force required to apply a constant amplitude oscillation will increase from gelification to full cure. Then, knowing the variation on dynamic force, the change in mechanical properties of the resin can be followed during polymerization. On the other hand, when the resin shrinks during cure, the instrument adjusts the position of the upper plate so that it is in continuous contact with the sample. Measuring the static position of the upper plate is then a direct evaluation of the volume changes occurred during resin polymerization. Fig.1. Novel thermo-mechanical cell HFC200. 3 Results and discussion The heat of reaction released by the polymer during curing is determined from the measured heat flux. If the diffusion of chemical species is neglected, the reaction rate is assumed to be a unique function of the degree of conversion α and temperature T [1]: t d d and (1) f T , dt dt dt 0 Fig.2. Comparison between degree of cure measure In the novel characterization cell described in this with DSC and DMA cell. work, the heat of reaction was measured with the heat flux sensors in close contact with the sample. As shown in Figure 3, at the early beginning of the The heat of reaction was computed as the average test, when the resin is fully liquid, a stiffness of 10 heat flow measured at the upper and lower plates of N/m is measured by the DMA-flux cell. This small the cell. An average heat of reaction of 337 J/g was stiffness is due to the moving liquid under dynamic measured for the DGBA system tested in this study, compaction. The stiffness increases linearly the first which is very similar to the value of 321 J/g 200 seconds up to 50 N/m corresponding to 20% of obtained with the Differential Scanning Calorimeter cure. At this stage the polymer chains start to form (DSC). Figure 2 illustrates the evolution of degree of an inter-connected tridimensional network resulting cure with time using both DMA cell and DSC in a quick increment of mechanical properties shown methods. The degree of cure evaluated with the DSC by sudden change to a steeper slope in stiffness. The technique (full line) arises before that of the DMA. Tan δ is the ratio between the stored and loss factors This time shift is not negligible and should therefore of the dynamic stiffness. The Tan δ in Figure 3 be compensated in the case of coupling shows a peak at 230 seconds representing a measurements from different devices. In Figure 2, maximum of the dissipating energy of the sample. the DMA and DSC curves show a similar slope This peak is associated to the gelification of the between 30 and 80% cure, however, there are
Recommend
More recommend