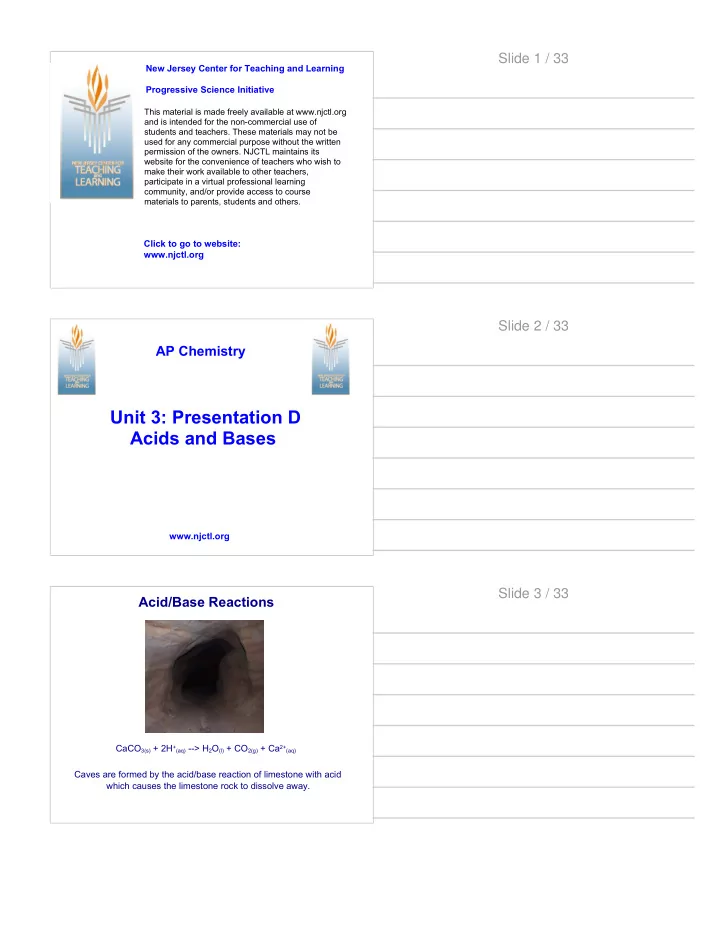

Slide 1 / 33 New Jersey Center for Teaching and Learning Progressive Science Initiative This material is made freely available at www.njctl.org and is intended for the non-commercial use of students and teachers. These materials may not be used for any commercial purpose without the written permission of the owners. NJCTL maintains its website for the convenience of teachers who wish to make their work available to other teachers, participate in a virtual professional learning community, and/or provide access to course materials to parents, students and others. Click to go to website: www.njctl.org Slide 2 / 33 AP Chemistry Unit 3: Presentation D Acids and Bases www.njctl.org Slide 3 / 33 Acid/Base Reactions CaCO 3(s) + 2H +(aq) --> H 2 O (l) + CO 2(g) + Ca 2+(aq) Caves are formed by the acid/base reaction of limestone with acid which causes the limestone rock to dissolve away.
Slide 4 / 33 Acid and Base Definitions The most widely used definition of acids and bases in the Bronsted- Lowry definition. ACID BASE H + Donor H + acceptor NH 4+(aq) + H 2 O (l) --> NH 3(g) + H 3 O +(aq) Acid: Base: H + Donor H + Acceptor Slide 5 / 33 Conjugate Acids and Bases After an acid donates an H+ ion, the resulting species can always behave as a base and grab the H+ back again. NH 4+(aq) + H 2 O <--> NH 3(g) + H 3 O +(aq) Base: Acid: H + Acceptor H + Donor Therefore, NH 3 acts as the conjugate base of NH 4+ and H 3 O + acts as the conjugate acid of H 2 O. NH 4+(aq) + H 2 O (l) <--> NH 3(g) + H 3 O +(aq) Slide 6 / 33 Conjugate Acids and Bases CN -(aq) --> HCN (aq) Predicting the base Conj. acid formula of a conjugate acid or HCO 3-(aq) --> CO 32-(aq) base involves acid Conj. base writing the formula of the species CH 3 NH 2(aq) --> CH 3 NH 3+(aq) produced after the base Conj. acid donation or acceptance of an CH 3 COOH (aq) --> CH 3 COO -(aq) H+ ion. Conj. base acid
Slide 7 / 33 Amphoteric Molecules Amphoteric molecules can behave as either acids or bases depending on their environment. Water is a classic example H 2 O donates H + accepts H + H 3 O + OH - Conj. acid Conj. base Whether water acts as an acid or base will depend on the relative "strength" of other acids or bases it is reacted with. Slide 8 / 33 Amphoteric Molecules Common amphoteric molecules are the conjugate bases of diprotic or polyprotic acids H 2 C 2 O 4(aq) HC 2 O 4-(aq) C 2 O 42-(aq) Acid Amphoteric Base H 3 PO 4(aq) H 2 PO 4-(aq) HPO 42-(aq) PO 43-(aq) Acid Amphoteric Amphoteric Base Slide 9 / 33 1 Which of the following is NOT an acid/base reaction? A HCN (aq) + H 2 O (l) --> CN -(aq) + H 3 O +(aq) B NH 3(aq) + HC 2 H 3 O 2(aq) --> NH 4+(aq) + C 2 H 3 O 2-(aq) C H +(aq) + H 2 O (l) --> H 3 O +(aq) D Pb 2+(aq) + 2I -(aq) --> PbI 2(s) E All are acid/base reactions
Slide 9 (Answer) / 33 1 Which of the following is NOT an acid/base reaction? A HCN (aq) + H 2 O (l) --> CN -(aq) + H 3 O +(aq) B NH 3(aq) + HC 2 H 3 O 2(aq) --> NH 4+(aq) + C 2 H 3 O 2-(aq) C H +(aq) + H 2 O (l) --> H 3 O +(aq) Answer D Pb 2+(aq) + 2I -(aq) --> PbI 2(s) D E All are acid/base reactions [This object is a pull tab] Slide 10 / 33 2 In which of the following reactions does the underlined substance behave as an acid? I. CH 3 NH 2(g) + H 2 O (l) --> CH 3 NH 3+(aq) + OH -(aq) A I only II. HCOOH (aq) + NH 2-(aq) --> HCOO -(aq) + NH 3(aq) III. HClO (aq) + OH- (aq) --> ClO -(aq) + H 2 O (l) B II only C III only D II and III only E I, II, and III Slide 10 (Answer) / 33 2 In which of the following reactions does the underlined substance behave as an acid? I. CH 3 NH 2(g) + H 2 O (l) --> CH 3 NH 3+(aq) + OH -(aq) A I only II. HCOOH (aq) + NH 2-(aq) --> HCOO -(aq) + NH 3(aq) III. HClO (aq) + OH- (aq) --> ClO -(aq) + H 2 O (l) B II only C III only Answer D D II and III only E I, II, and III [This object is a pull tab]
Slide 11 / 33 3 What would be the expected products of the following reaction... H 2 PO 4-(aq) + OH -(aq) --> ____________ A H 3 PO 4(aq) + H 2 O (l) B H 2 PO 42-(aq) + H 2 O (l) C HPO 4-(aq) + H 3 O +(aq) D HPO 42-(aq) + H 2 O (l) E None of these Slide 11 (Answer) / 33 3 What would be the expected products of the following reaction... H 2 PO 4-(aq) + OH -(aq) --> ____________ A H 3 PO 4(aq) + H 2 O (l) B H 2 PO 42-(aq) + H 2 O (l) Answer D C HPO 4-(aq) + H 3 O +(aq) D HPO 42-(aq) + H 2 O (l) E None of these [This object is a pull tab] Slide 12 / 33 4 Which of the following is the conjugate acid of the chlorite ion (ClO 2 -)? A HClO 2 B HClO 2+ C HClO D HClO 3 E HClO 2-
Slide 12 (Answer) / 33 4 Which of the following is the conjugate acid of the chlorite ion (ClO 2 -)? A HClO 2 Answer B HClO 2+ A C HClO D HClO 3 [This object is a pull tab] E HClO 2- Slide 13 / 33 5 Which of the following has the acid paired with it's appropriate conjugate base? I. HPO 42-(aq) /PO 42-(aq) A I only II. HBrO 2(aq) /BrO -(aq) B II only III. H 2 CrO 4(aq) /HCrO 4-(aq) C III only D I and III only E I and II only Slide 13 (Answer) / 33 5 Which of the following has the acid paired with it's appropriate conjugate base? I. HPO 42-(aq) /PO 42-(aq) A I only II. HBrO 2(aq) /BrO -(aq) B II only III. H 2 CrO 4(aq) /HCrO 4-(aq) Answer C III only C D I and III only E I and II only [This object is a pull tab]
Slide 14 / 33 6 Which of the following would be amphoteric? A CO 32-(aq) B HC 2 H 3 O 2(aq) C HC 2 O 4-(aq) D F -(aq) E H 3 O +(aq) Slide 14 (Answer) / 33 6 Which of the following would be amphoteric? A CO 32-(aq) B HC 2 H 3 O 2(aq) Answer C C HC 2 O 4-(aq) D F -(aq) [This object is a pull tab] E H 3 O +(aq) Slide 15 / 33 Strong Acids Strong acids dissociate 100% in aqueous solutions thereby donating ALL of their H+ ions. Therefore, much like a soluble ionic compound, any strong acid must be written as being broken up into it's ions. Strong Acid Written as... HCl (aq) H +(aq) + Cl -(aq) HNO 3(aq) H +(aq) + NO 3-(aq) H 2 SO 4(aq) 2H +(aq) + SO 42-(aq) HClO 4(aq) H +(aq) + ClO 4-(aq) HBr (aq) H +(aq) + Br -(aq) HI (aq) H +(aq) + I -(aq)
Slide 16 / 33 Strong Acids Conjugate bases of strong acids are not able to accept H+ ions back and therefore do not participate in acid/base reactions. H +(aq) + NO 3-(aq) + NH 3(aq) --> NH 4+(aq) H +(aq) + NH 3(aq) --> NH 4+(aq) H +(aq) + Br -(aq) + ClO -(aq) --> HClO (aq) H +(aq) + ClO -(aq) --> HClO (aq) Slide 17 / 33 Writing Acid/Base Reactions First write the correct formulas for all species involved, breaking compounds into ions if soluble ionics or strong acids. Example: Aqueous solutions of methlyamine (CH3NH2) and hydroiodic acid are mixed. CH 3 NH 2(aq) + H +(aq) + I -(aq) --> Predict products based on acid/base principles. CH 3 NH 2(aq) + H +(aq) + I -(aq) --> CH 3 NH 3+(aq) CH 3 NH 2(aq) + H +(aq) --> CH 3 NH 3+(aq) Slide 18 / 33 7 Which of the following species would be an extremely weak base? A Br - B F - C NO 2- D ClO 3- E NH 3
Slide 18 (Answer) / 33 7 Which of the following species would be an extremely weak base? A Br - Answer B F - A C NO 2- D ClO 3- [This object is a pull tab] E NH 3 Slide 19 / 33 8 Why are strong acids written as separate ions? A Strong acids are dangerous B Strong acids have strong conjugate bases C Strong acids do not have conjugate bases D Strong acids donate all of their H+ ions E None of these Slide 19 (Answer) / 33 8 Why are strong acids written as separate ions? A Strong acids are dangerous Answer B Strong acids have strong conjugate bases D C Strong acids do not have conjugate bases D Strong acids donate all of their H+ ions [This object is a pull tab] E None of these
Slide 20 / 33 9 Which of the following is NOT a strong acid? A HNO 3 B HF C HBr D HClO 4 E H 2 SO 4 Slide 20 (Answer) / 33 9 Which of the following is NOT a strong acid? A HNO 3 B HF Answer C HBr B D HClO 4 E H 2 SO 4 [This object is a pull tab] Slide 21 / 33 10 Which of the following best represents the balanced equation for the acid/base reaction of aqueous potassium hydroxide and chromic acid (H 2 CrO 4 )? A OH - + H 2 CrO 4 → H 2 O + CrO 42- B 2K + + H 2 CrO 4 → 2H + + K 2 CrO 4 C 2OH - + H 2 CrO 4 → 2H 2 O + CrO 42- D 2K + + H 2 CrO 4 → 2KH + CrO 42- E None of these
Slide 21 (Answer) / 33 10 Which of the following best represents the balanced equation for the acid/base reaction of aqueous potassium hydroxide and chromic acid (H 2 CrO 4 )? A OH - + H 2 CrO 4 → H 2 O + CrO 42- B 2K + + H 2 CrO 4 → 2H + + K 2 CrO 4 Answer C C 2OH - + H 2 CrO 4 → 2H 2 O + CrO 42- D 2K + + H 2 CrO 4 → 2KH + CrO 42- E None of these [This object is a pull tab] Slide 22 / 33 11 Which of the following best represents the balanced equation for the acid/base reaction if solid strontium hydroxide is added to a solution of acetic acid (HC 2 H 3 O 2 )? A Sr(OH) 2 + 2HC 2 H 3 O 2 → 2C 2 H 3 O 2- + Sr 2+ + 2H 2 O B 2OH - + 2HC 2 H 3 O 2 → 2C 2 H 3 O 2- + 2H 2 O C Sr(OH) 2 + 2C 2 H 3 O 2- → Sr(C 2 H 3 O 2 ) 2 + 2OH - D Sr(OH) 2 + HC 2 H 3 O 2 → C 2 H 3 O 2- + Sr 2+ + H 2 O E None of these Slide 22 (Answer) / 33 11 Which of the following best represents the balanced equation for the acid/base reaction if solid strontium hydroxide is added to a solution of acetic acid (HC 2 H 3 O 2 )? A Sr(OH) 2 + 2HC 2 H 3 O 2 → 2C 2 H 3 O 2- + Sr 2+ + 2H 2 O Answer B 2OH - + 2HC 2 H 3 O 2 → 2C 2 H 3 O 2- + 2H 2 O A C Sr(OH) 2 + 2C 2 H 3 O 2- → Sr(C 2 H 3 O 2 ) 2 + 2OH - D Sr(OH) 2 + HC 2 H 3 O 2 → C 2 H 3 O 2- + Sr 2+ + H 2 O [This object is a pull tab] E None of these
Recommend
More recommend