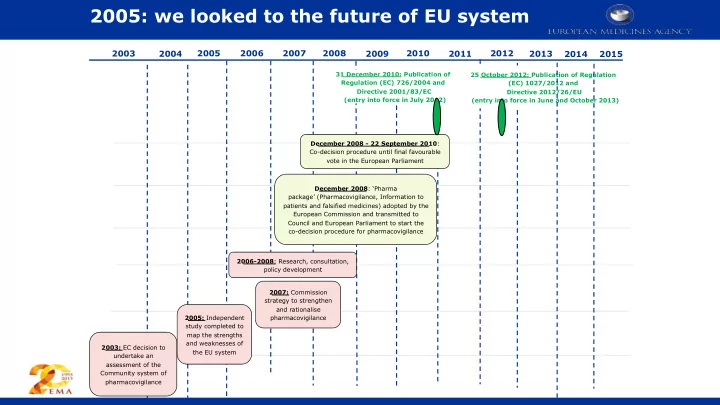

2005: we looked to the future of EU system 2008 2010 2012 2003 2004 2005 2006 2007 2009 2011 2013 2014 2015 31 December 2010: Publication of 25 October 2012: Publication of Regulation Regulation (EC) 726/2004 and (EC) 1027/2012 and Directive 2001/83/EC Directive 2012/26/EU (entry into force in July 2012) (entry into force in June and October 2013) December 2008 - 22 September 2010 : Co-decision procedure until final favourable vote in the European Parliament December 2008 : ‘Pharma package’ (Pharmacovigilance, Information to patients and falsified medicines) adopted by the European Commission and transmitted to Council and European Parliament to start the co-decision procedure for pharmacovigilance 2006-2008 : Research, consultation, policy development 2007: Commission strategy to strengthen and rationalise 2005: Independent pharmacovigilance study completed to map the strengths and weaknesses of 2003: EC decision to the EU system undertake an assessment of the Community system of pharmacovigilance 1
Future of pharmacovigilance in 2015: Back to the future 2
Back to the future of pharmacovigilance 3
The future of pharmacovigilance Thanks to: Munir Pirmohamed • Corinne de Vries • Steven Evans • Xavier Kurz • June Raine • Georgy Genov • Almath Spooner • • Ana Hidalgo Bert Leufkens • • Fergus Sweeney Brian Edwards • • Hans-Georgy Eichler Peter Backman • • Guido Rasi Mick Foy • 4
The future of pharmacovigilance In this presentation: Introduction: a brief moment in time • Axes of influence • Societal opportunities • Technological opportunities • Scientific opportunities • The future of pharmacoviglance • 5
The future of pharmacovigilance In this presentation: Introduction: a brief moment in time • Axes of influence • Societal opportunities • Technological opportunities • Scientific opportunities • A call to arms for health and innovation • 6
The future of pharmacovigilance: a brief moment in time Past From individual cases to pharmacoepidemiology • From local to international • From exclusive to inclusive • From opaque to transparent • From pursued to require • From safety to benefit risk • 7
The future of pharmacovigilance: a brief moment in time Present In many countries and regions: Lifecycle approach • Planned data collection and risk minimisation • Some integration of benefits and risks • Clear roles and responsibilities • Quality systems approach • Engagement with patients and across disciplines (HTA) • 8
The future of pharmacovigilance: Looking forward Planned, integrated lifecycle drug development and • surveillance Utilisation of validated scientific methods , • Real world data : quality, accessible, timely • Best use of technology • Meeting expectations of a changing society • Delivering timely access for patients to safe and effective • medicines Making an impact on health promotion, protection and • innovation 9
The future of pharmacovigilance: a brief moment in time Future Achieving the vision • ….through multiple small steps 10
The future of pharmacovigilance In this presentation: Introduction: a brief moment in time • Axes of influence • Societal opportunities • Technological opportunities • Scientific opportunities • A call to arms for health and innovation • 11
The future of pharmacovigilance: Axes of influence 1. Time: past, present, future 2. Geographic: local vs global 3. Sectors: pharmaceutical, devise, healthcare systems, patients safety 4. Economic (cost): healthcare, medicines, studies, adverse reactions, unmet need 5. Political: peace, healthcare system, regulation, functioning market 6. Societal: more coming 7. Technological: more coming 8. Scientific: more coming 12
Time: European Anniversary year 2015: 50 years of EU regulation 20 years of EMA 5 years since adoption of Pharmacovig legislation 3 years of PRAC 13
Geographic 14
Sectors 15
Economics: healthcare, medicines, studies, unmet need adverse reactions Medicines save lives and relieve suffering, but: • 5% of all hospital admissions are for ADRs, • 5% of all hospital patients suffer an ADR, • ADRs are the 5 th most common cause of hospital death • Estimated 197,000 deaths per year in EU from ADRs • EU Societal cost of ADRs Euro 79 Billion / year 16
Economics 17
Political 18
The future of pharmacovigilance In this presentation: Introduction: a brief moment in time • Axes of influence • Societal opportunities • Technological opportunities • Scientific opportunities • A call to arms for health and innovation • 19
The future of pharmacovigilance: Societal opportunities Patient and healthcare professionals ready to engage: reporting, assessing, • values, deciding, enacting, feeding back Demographics: aging population, new arrivals in the EU • Demand for evidence based for use of medicines in pregnancy, and children • 24-hour news cycle, web-based communications • Demand to fulfil unmet medical needs • Demands for simplification • Recognition that collaboration can deliver for health, through sharing • expertise, data, new uses for old drugs Better and more accessible information for decision support • 20
More and better patient engagement 21
Demographics: change is sometimes difficult to predict. 22
Special populations: good work done but so much more to deliver
Unmet medical need: Adaptive pathways concept ("widening of the indication") Time Final target indication in 1st approval 2nd approval blue, patient group with highest need in red the sponsor could follow two strategies 1st approval
Simplification 25
26
Collaboration: to achieve more and better 27
Decision cycle EMA Committee raises issue Measure Meeting to effectiveness consider further of action research Information from Regulatory network Communication clinical use study Results to +/- Regulatory EMA action Committee Regulatory assessment 28 Adapted from Arlett et al. Pharmacoepidemiol Drug Saf. 2014;23(4):431-4
The future of pharmacovigilance In this presentation: Introduction: a brief moment in time • Axes of influence • Societal opportunities • Technological opportunities • Scientific opportunities • A call to arms for health and innovation • 29
The future of pharmacovigilance: Technological opportunities Product types e.g. biologicals, advanced therapy medicinal products, combination • products, vaccines Tracing the distribution of medicines • Social media: linkage, privacy, quality. Where to focus, how can the methodologists • help us? m-health: smart phones for case reporting, for patient led cohorts, for recruitment, • to support health decision-making. m-health: patients self monitoring using mobile devices • 2025 “Oyster card” for health (not a new idea, but getting closer to possible) • 30
Monitoring specific product types 31
EU counterfeit medicines legislation foresees tracking of prescription products 32
Smartphones and mobile apps 1.75 billion smartphones in • 1.3 million apps available for • use worldwide android users (1.2 million for iOS) 34.6 million in the UK • Around 6,000 health related • 62% of UK adults and 53% • apps of households have a smartphone (24% also UK NHS has its own app store • have a tablet)
Social media 1.35 billion active Facebook • 284 million active Twitter users • users 15 million in the UK • 31.5 million in the UK • 80% active on mobile • 680 million on mobile devices • 500 million tweets per day • 48% log on every day • worldwide 25-34 largest age group •
Social media as an opportunity and challenge 35
m-health: patients self monitoring using mobile devices 36
Are we heading for patient health cards? 37
The future of pharmacovigilance In this presentation: Introduction: a brief moment in time • Axes of influence • Societal opportunities • Technological opportunities • Scientific opportunities • A call to arms for health and innovation • 38
The future of pharmacovigilance: Scientific opportunities 1 Adverse Drug Reaction Reports: long live ADR reporting; improve quantity and • quality of reporting; link data to EHR and biological archives Registries / cohorts – key for rare diseases, + hospital and specialist use. Need for • better tools to support: protocols, standard data fields, cheap and accessible data collection Epidemiological methods – PROTECT, ENCePP, EU-ADR, OMOP – infrastructure for • studies (data, access, governance, funding) Signals: combine data sources; multiple imputation for missing data; implement • best established methods; use EHR for some types of events; outliers in clinical trials 39
The future of pharmacovigilance: Scientific opportunities 2 Genomics: eliglustat; abacavir; warfarin – better labelling of genomics to support • decisions; precision medicine techniques; individual patient BR decisions; Integrating benefit and risk (effectiveness and safety) monitoring and rapid-cycle • analytics Benefit risk assessment methods and decisions • Impact: ensuring we measure what works and what does not and that we • continuously improve 40
Recommend
More recommend