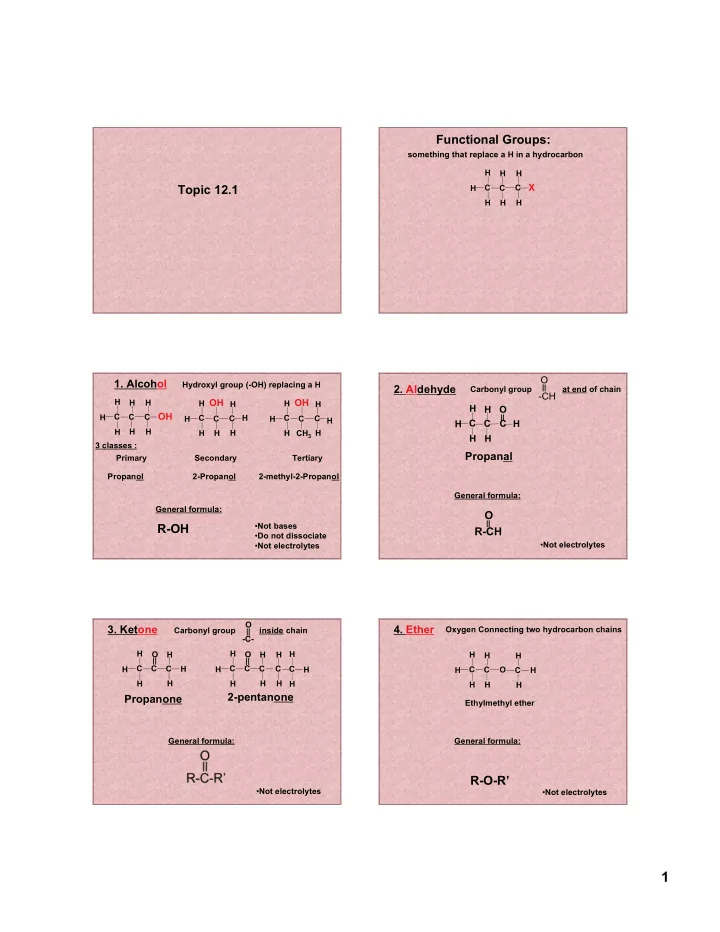

Functional Groups: something that replace a H in a hydrocarbon H H H X Topic 12.1 C C C H H H H O 1. Alcohol Hydroxyl group (-OH) replacing a H 2. Aldehyde Carbonyl group at end of chain -CH H H OH H OH H H H H H H O OH H C C C C C C H C C C H H H H C C C H H H H H H H H CH 3 H H H 3 classes : Propanal Primary Secondary Tertiary Propanol 2-Propanol 2-methyl-2-Propanol General formula: General formula: O R-OH • Not bases R-CH • Do not dissociate • Not electrolytes • Not electrolytes O 3. Ketone 4. Ether Carbonyl group inside chain Oxygen Connecting two hydrocarbon chains -C- H H O H O H H H H H H C C C H C C C C C H H H H C C O C H H H H H H H H H H 2-pentanone Propanone Ethylmethyl ether General formula: General formula: R-O-R’ • Not electrolytes • Not electrolytes 1
O O 5. Organic Acids 6. Esters Carboxyl group of chain Carboxyl group in middle of chain -C-OH -C-O H H H O H O H H H H C C C OH C C C O C H H C C C H H H H H H H H H Propanoic Acid Comes from Comes from acid alcohol General formula: Butyl Propanoate O O R-C-OH Acidic hydrogen (H + ) • Weak acids R-C-O-R’ General formula: • Do dissociate • weak electrolytes • Not electrolytes 8. Amino acids = have an amino and a carboxyl group 7. Amines = organic compounds based on ammonia : N H H H 2 H 3 C CH 3 H O H H C C N H 2 H N C C OH : H N C H CH 2 H H H CH 3 R Methylamine Triethylamine Methanamine Triethanamine • weak bases • Split water General formula: R-NH 2 • weak electrolytes Structure general formula type of compound name Structure general formula type of compound name O O H 2 H 2 H 2 O H 2 H 2 C C C O ester propylpentanoate org. acid heptanoic acid 1 CH 3 7 H 3 C C C H 3 C C C C O C R-C-O-R’ H 2 H 2 R-C-O-H C H 2 H 2 OH H 2 O O H 2 H 2 H 2 H 2 1° alcohol 1-pentanol ester ethyl pentanoate 2 C C OH 8 R-OH H 3 C C C C C C C O CH 3 H 3 C R-C-O-R’ H 2 H 2 H 2 H 2 O H 2 CH 3 H 2 H 2 ketone 3-heptanone 3° alcohol 2-methyl 2-heptanol 3 H 3 C C CH 3 9 R-OH C OH C H 3 C C C C C C H 2 H 2 H 2 CH 3 H 2 H 2 O O O CH 3 O 4 10 H 2 aldehyde pentanal aldehyde 5-methyl hexanal H 3 C C C C CH C C CH H 2 H 2 H 2 R-CH R-CH H 3 C H C C H 2 H 2 H 2 H 2 H 2 O 5 11 H 2 C C C ether butyl ethyl ether ketone 2-pentanone R-O-R’ H 3 C O C CH 3 C H 2 H 3 C C CH 3 H 2 H 2 H 2 H 2 H 2 H 2 H 2 2° alcohol 3-heptanol ether propyl pentyl ether 6 C H C C R-OH 12 R-O-R’ H 3 C C C CH 3 H 3 C C C C CH 3 H 2 C C O C OH H 2 H 2 H 2 2
Structure general formula type of compound name O O org. acid hexanoic acid H 2 H 2 11 C C H 3 C C C OH R-C-OH H 2 H 2 OH 12 H 2 H 2 3° alcohol 4-methyl 4-octanol R-OH C C CH 3 H 3 C C C C CH 3 H 2 H 2 H 2 3
Recommend
More recommend